Validation of an RP-HPLC Method to Determine Rupatadine as a Fumarate in Pharmaceutical Dosage Form
Abstract
 Abstract Views: 0
Abstract Views: 0
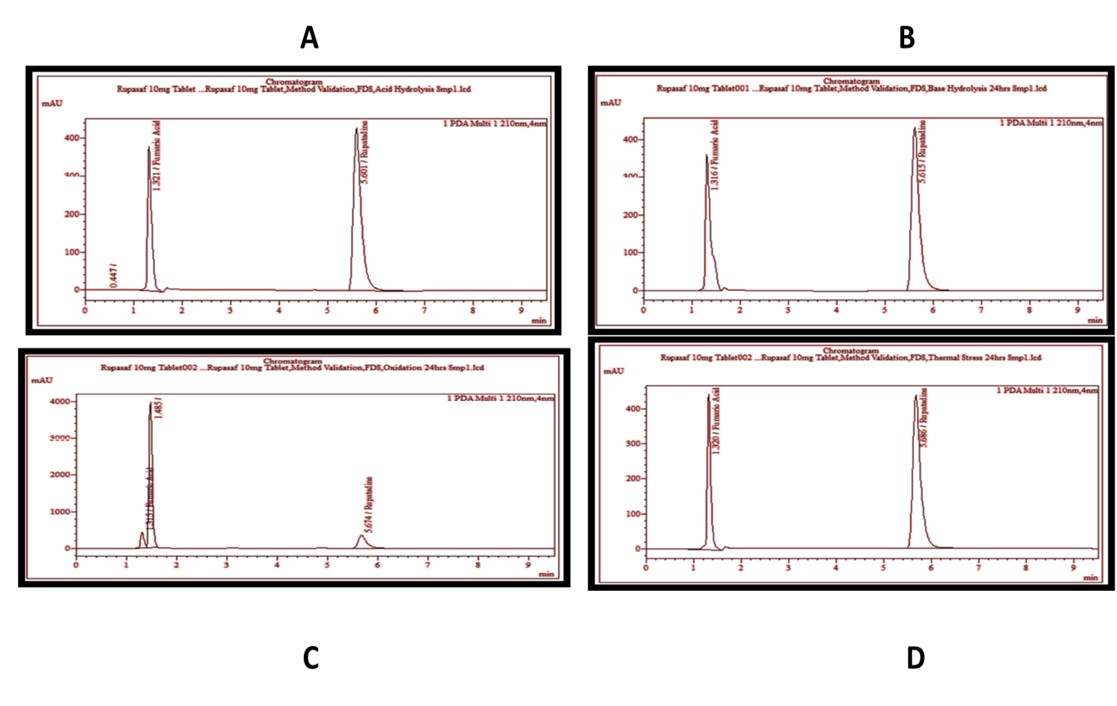
Rupatadine (RUP) is a second generation antihistamine drug and an agonist of platelets activating factor. Literature does not report any specific method for the determination of RUP. Therefore, a Reverse Phase High Performance Liquid Chromatography (RP-HPLC) method was developed in this research for the quantitative determination of RUP in pharmaceutical dosage form. For this purpose, a mixture of monosodium phosphate buffer and acetonitrile 80:20 v/v was used as mobile phase, flowing at a rate of 1.0 ml/min. A well-characterized reference material of RUP with a potency of 99.68% (as is basis) was used throughout the study. RUP for system suitability chemical related substances (CRS) (containing impurities A and B) was also used in this study for the identification of fumaric acid, impurity A, impurity B, and RUP in the pharmaceutical dosage form. Method validation was performed by the preliminary analysis of the standard sample and by performing recovery studies. The method was found to be linear with regression line y = 95464x -6164, having correlation 1.0000, in the range of 17.04-85.20 µg/ml. Moreover, percentage recovery of RUP was found to be in the range of 99.08 to 100.25. The limit of detection (LOD) was found to be 0.63 µg/ml and the limit of quantification (LOQ) was found to be 1.91µg/ml. It can be inferred from the obtained results that the developed method is simple, linear, precise, accurate, and robust. Therefore, it can be employed for the quantitative determination of RUP in the pharmaceutical dosage form.
Downloads
References
Brayfield A, Martindale: the complete drug reference. Pharmaceutical Press; 2017.
Tian X, Ma W, Yusuf B, Su B, Hu J, Zhang T. Assessment of the efficacy of the antihistamine drug rupatadine used alone or in combination against mycobacteria. Pharmaceutics. 2024;16(8):e1049. https://doi.org/10.3390/pharmaceutics16081049
Alexander M, Alexander J. Rupatadine-Induced nocturnal dyskinesia and paroxysmal sleep terrors in a 72-Year-Old Male with Anaphylaxis. Ann Allergy Asthma Immunol. 2024;133(6):eS109. https://doi.org/10.1016/j.anai.2024.08.476
Winnicka K. Utilization of ethylcellulose microparticles with rupatadine fumarate in designing orodispersible minitablets with taste masking effect. Materials. 2020;13(12):e2715. https://doi.org/10.3390/ma13122715
Joint Formulary Committee. BNF 78: September 2019–March 2020. BMJ Group/Pharmaceutical Press; 2019.
Malavige GN, Wijewickrama A, Fernando S, et al. A preliminary study on efficacy of rupatadine for the treatment of acute dengue infection. Sci Rep. 2018;8:e3857. https://doi.org/10.1038/s41598-018-22285-x
Nogueira DR, D’Avila FB, Rolim CMB, Dalmora SL. Development and validation of a stability-indicating LC method for the determination of rupatadine in pharmaceutical formulations. Chroma. 2007;66:915–919. https://doi.org/10.1365/s10337-007-0426-0
Olechno K, Maciejewski B, Głowacz K, et al. Orodispersible films with rupatadine fumarate enclosed in ethylcellulose microparticles as drug delivery platform with taste-masking effect. Materials. 2022;15(6):e2126. https://doi.org/10.3390/ma15062126
Ellis AK, Connors L, Francoeur MJ, Mack DP. Rupatadine to prevent local allergic reactions to sublingual allergy immunotherapy: a case series. Allergy Asthma Clin Immunol. 2021;17:e125. https://doi.org/10.1186/s13223-021-00630-6
Shirkhedkar AS, Thorve RT, Fursule RF, Surana SS. Development and validation of a stability-indicating HPTLC method for analysis of rupatadine fumarate in the bulk drug and tablet dosage form. Acta Chromatogr. 2008;20(3):423–437. https://doi.org/10.1556/achrom.20.2008.3.9
Nogueira DR, Sangoi MS, da Silva LM, Todeschini V, Dalmoraet SL. Determination of rupatadine in pharmaceutical formulations by a validated stability‐indicating MEKC method. J Sep Sci. 2008;31(16‐17):3098–3105. https://doi.org/10.1002/jssc.200800254
Goyal A, Sharma C, Singh G. Development of UV and Visible Spectrophotometric methods for estimation of rupatadine fumarate from tablet formulation. Int J Pha Res Dev. 2010;2(4):e14.
Rele R, Desai P, Sawant S. Simple extractive spectrophotometric determination of rupatadine as rupatadine fumarate from pharmaceutical formulation. Int J Chem Sci. 2010;8(1):22–28.
Rele RV, Mahimkar SA, Sawant SA. A validated simple titrimetric method for the quantitative determination of rupatadine as rupatadine fumarate from pharmaceutical dosages. Anal Chem Indian J. 2009;8:561–564.
Trivedi HK, Patel MC. Development of a stability-indicating RP-HPLC method for the determination of rupatadine and its degradation products in solid oral dosage form. Sci Pharm. 2012;80(4):889–902. https://doi.org/10.3797/scipharm.1208-10
Cai Y, Mclaughlin M, Zhang K. Advancing the FDA/office of regulatory affairs mycotoxin program: new analytical method approaches to addressing needs and challenges. J AOAC Int. 2020;103(3):705–709. https://doi.org/10.1093/jaocint/qsz007
Guideline IHT. Q2 (R1) Validation of analytical procedures: text and methodology. In: Teasdale A, Elder D, Nims RW, eds. ICH Quality Guidelines: An Implementation Guide. John Wiley & Sons, Inc; 2005: 128–166.
Sukumar V, Chinnusamy S, Chanduluru HK, Rathinam S. Method development and validation of Atorvastatin, Ezetimibe and Fenofibrate using RP-HPLC along with their forced degradation studies and greenness profiling. Green Chem Lett Rev. 2023;16(1):e2198651. https://doi.org/10.1080/17518253.2023.2198651
Al Bratty M, Thangavel N, Peraman R, et al. HPLC–DAD method for investigating pantoprazole for its stress-dependent degradation by photolysis and oxidation. Acta Chromatogr. 2020;32(4):247–255. https://doi.org/10.1556/1326.2019.00709
Al-Sanea MM, Gamal M. Critical analytical review: rare and recent applications of refractive index detector in HPLC chromatographic drug analysis. Microchem J. 2022;178:e107339. https://doi.org/10.1016/j.microc.2022.107339
Abba S, Usman A, Selin I. Simulation for response surface in the HPLC optimization method development using artificial intelligence models: a data-driven approach. Chem Intell Lab Sys. 2020;201:e104007. https://doi.org/10.1016/j.chemolab.2020.104007
Kotani A, Watanabe R, Hayashi Y, Hakamata H. Chemometric evaluations of repeatability and detection limit in high-performance liquid chromatography with electrochemical detection. J Chromatogr A. 2022;1673:e463075. https://doi.org/10.1016/j.chroma.2022.463075
Woziński M, Greber KE, Pastewska M, et al. Modification of gradient HPLC method for determination of small molecules' affinity to human serum albumin under column safety conditions: robustness and chemometrics study. J Pharm Biomed Anal. 2024;239:e115916. https://doi.org/10.1016/j.jpba.2023.115916
Amer M, Habib AA, Hammad S, Kamal A. Review on strategies for analysis of some structurally related pharmaceutical compounds: approach to forced degradation, degradation kinetics and impurity profiling of drugs. J Adv Med Pharm Res. 2025;6(1):32–47. https://doi.org/10.21608/jampr.2025.342078.1084
Salomone A, Di Corcia D, Negri P, et al. Targeted and untargeted detection of fentanyl analogues and their metabolites in hair by means of UHPLC-QTOF-HRMS. Anal Bioanal Chem. 2021;413:225–233. https://doi.org/10.1007/s00216-020-02994-x
Hanif S, Syed MA, Rashid AJ, et al. Validation of a novel RP-HPLC technique for simultaneous estimation of lignocaine hydrochloride and tibezonium iodide: greenness estimation using AGREE penalties. Molecules. 2023;28(8):e3418. https://doi.org/10.3390/molecules28083418
González-González O, Ramirez IO, Ramirez BI, et al. Drug stability: ICH versus accelerated predictive stability studies. Pharmaceutics. 2022;14(11):e2324. https://doi.org/10.3390/pharmaceutics14112324
Copyright (c) 2025 Qazi Amir Ijaz, Mohammad Ibrahim, Yasir Mehmood, Bilal Khalid, Muhammad Affan, Naila Abdul Sattar

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution (CC-BY) 4.0 License that allows others to share the work with an acknowledgement of the work’s authorship and initial publication in this journal









