Cubosomes in Drug Delivery: Exploring Their Potential for Advanced Therapeutic Applications
Abstract
 Abstract Views: 0
Abstract Views: 0
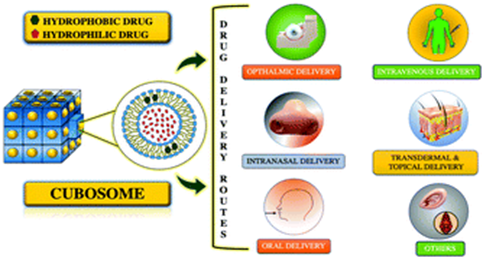
Cubosomes have become a subject of increasing significance because of their unique three-dimensional nano cubic lattice structure, which is composed of self-assembling lipid molecules like monoolein. A wide range of therapeutic agents, involving both hydrophilic and hydrophobic drugs, can be encapsulated within this stable and water-dispersible structure. Cubosomes' unique cubic lattice also facilitates surface modifications and lipid content adaptation, improving drug stability and resistance to degradation. This enables precise control over the kinetics of drug release and provides an intriguing framework for the development of controlled drug release systems. As a result, cubosomes can improve therapeutic efficacy while minimizing side effects of drugs molecules. In conclusion, this review explores the potential uses of cubosomes and highlights the specifics that emphasize their significance in improving drug delivery methods.
Downloads
References
Emeje MO, Ifeoma CO, Ekaete IA, Sabinus IO. Nanotechnology in drug delivery. In: Sezer AD, ed. Recent Advances in Novel Drug Carrier Systems. InTechOpen; 2012:69–106.
Varghese R, Salvi S, Sood P, Karsiya J, Kumar D. Cubosomes in cancer drug delivery: a review. Colloid Interface Sci Commun. 2021;46:e100561. https://doi.org/10.1016/j.colcom.2021.100561
Bahman MA. Investigating Liquid Crystal Nanoparticles for Placental Drug Delivery [dissertation]. Manchester: The University of Manchester; 2019.
Barriga HM, Holme MN, Stevens MM. Cubosomes: the next generation of smart lipid nanoparticles? Angew Chem Int Ed. 2019;58(10):2958–2978. https://doi.org/10.1002/anie.201804067
Varghese R, Salvi S, Sood P, Kulkarni B, Kumar D. Cubosomes in cancer drug delivery: a review. Colloid Interface Sci Commun. 2022;46:e100561. https://doi.org/10.1016/j.colcom.2021.100561
Lemes AC, Sala L, Ores JdC, Braga ARC, Egea MB, Fernandes KF. A review of the latest advances in encrypted bioactive peptides from protein-rich waste. Int J Mol Sci. 2016;17(6):e950. https://doi.org/10.3390/ijms17060950
Karami Z, Hamidi M. Cubosomes: remarkable drug delivery potential. Drug Discov Today. 2016;21(5):789–801. https://doi.org/10.1016/j.drudis.2016.01.004
Chountoulesi M, Pippa N, Pispas S, et al. Cubic lyotropic liquid crystals as drug delivery carriers: physicochemical and morphological studies. Int J Pharm. 2018;550(1–2):57–70. https://doi.org/10.1016/j.ijpharm.2018.08.003
Sivadasan D, Sultan MH, Alqahtani SS, Javed S. Cubosomes in drug delivery—a comprehensive review on its structural components, preparation techniques and therapeutic applications. Biomedicines. 2023;11(4):e1114. https://doi.org/10.3390/biomedicines11041114
Mukesh A, Shukla K, Pratap S. A comprehensive review on cubosomes. Int J Pharm Pharm Res. 2022;26(1):261–271.
Garg G, Saraf S, Saraf S. Cubosomes: an overview. Biol Pharm Bull. 2007;30(2):350–353. https://doi.org/10.1248/bpb.30.350
Zhao XY, Zhang J, Zheng LQ, Li DH. Studies of cubosomes as a sustained drug delivery system. J Dispersion Sci Technol. 2005;25(6):795–799. https://doi.org/10.1081/DIS-200035589
Dully M, Ceresnakova M, Murray D, Soulimane T, Hudson SP. Lipid cubic systems for sustained and controlled delivery of antihistamine drugs. Mol Pharm. 2021;18(10):3777–3794. https://doi.org/10.1021/acs.molpharmaceut.1c00279
Thomas A, Varghese J, Raju SP, Das C, Abraham E. Cubosomes—a novel drug delivery system. J Glob Trends Pharm Sci. 2017;8(4):4718–4727.
Dhadwal A, Sharma DR, Pandit V, Ashawat MS, Kumar P. Cubosomes: a novel carrier for transdermal drug delivery. J Drug Deliv Ther. 2020;10(1):123–130.
Spicer PT, Hayden KL, Lynch ML, Ofori-Boateng A, Burns JL. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir. 2001;17(19):5748–5756. https://doi.org/10.1021/la010161w
Rizwan SB, Boyd BJ. Cubosomes: structure, preparation and use as an antigen delivery system. In: Foged C, Rades T, Perrie Y, Hook S, eds. Subunit Vaccine Delivery. Springer Nature Link; 2015:125–140.
Patond VB, Ghonge AB, Narkhede MB. Cubosome—a review. Int J Trend Sci Res Dev. 2020;4(4):1116–1120.
Bryant SJ, Bathke EK, Edler KJ. Bottom-up cubosome synthesis without organic solvents. J Colloid Interface Sci. 2021;601:98–105. https://doi.org/10.1016/j.jcis.2021.05.072
Laya P, Bhattacharya S, Prajapati B. Cubosomes. In: Prajapati B, Patel J, eds. Lipid-Based Drug Delivery Systems. Jenny Stanford Publishing; 2020:147–183.
Sharma P, Dhawan S, Nanda S. Cubosome: a potential liquid crystalline carrier system. Curr Pharm Des. 2020;26(27):3300–3316. https://doi.org/10.2174/1381612826666200617162424
Mertins O, Mathews PD, Angelova A. Advances in the design of pH-sensitive cubosome liquid crystalline nanocarriers for drug delivery applications. Nanomaterials. 2020;10(5):e963. https://doi.org/10.3390/nano10050963
Palma AS, Casadei BR, Lotierzo MC, de Castro RD, Barbosa LRS. A short review on the applicability and use of cubosomes as nanocarriers. Biophys Rev. 2023;15:553–567. https://doi.org/10.1007/s12551-023-01089-y
Lombardo D, Kiselev MA. Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics. 2022;14(3):e543. https://doi.org/10.3390/pharmaceutics14030543
Holland JW, Cullis PR, Madden TD. Poly(ethylene glycol)−lipid conjugates promote bilayer formation in mixtures of non-bilayer-forming lipids. Biochemistry. 1996;35(8):2610–2617. https://doi.org/10.1021/bi951999j
Leung AK, Hafez IM, Baoukina S, et al. Lipid nanoparticles containing siRNA synthesized by microfluidic mixing exhibit an electron-dense nanostructured core. J Phys Chem C. 2012;116(34):18440–18450. https://doi.org/10.1021/jp303267y
Rajesh S, Leiske MN, Leitch V, et al. Lipidic poly(2-oxazoline)s as PEG replacement steric stabilisers for cubosomes. J Colloid Interface Sci. 2022;623:1142–1150. https://doi.org/10.1016/j.jcis.2022.04.158
Siekmann B, Bunjes H, Koch MH, Westesen K. Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride–water phases. Int J Pharm. 2002;244(1-2):33–43. https://doi.org/10.1016/S0378-5173(02)00298-3
Wu H, Li J, Zhang Q, et al. A novel small Odorranalectin-bearing cubosomes: Preparation, brain delivery, and pharmacodynamic study on amyloid-β25–35-treated rats following intranasal administration. Eur J Pharm Biopharm. 2012;80(2):368–378. https://doi.org/10.1016/j.ejpb.2011.10.012
Pitzalis P, Monduzzi M, Krog N, Larsson H, Ljusberg-Wahren H, Nylander T. Characterization of the liquid−crystalline phases in the glycerol monooleate/diglycerol monooleate/water system. Langmuir. 2000;16(15):6358–6365. https://doi.org/10.1021/la0002031
Narayanan T, Konovalov O. Synchrotron scattering methods for nanomaterials and soft matter research. Materials. 2020;13(3):e752. https://doi.org/10.3390/ma13030752
Kwon TK, Kim JC. In vitro skin permeation and anti-atopic efficacy of lipid nanocarriers containing water-soluble extracts of Houttuynia cordata. Drug Dev Ind Pharm. 2014;40(10):1350–1357. https://doi.org/10.3109/03639045.2013.819883
Omar S, Ismail A, Hassanin K, Hamdy S. Formulation and evaluation of cubosomes as a skin retentive system for topical delivery of clotrimazole. J Adv Pharm Res. 2019;3(2):68–82. https://dx.doi.org/10.21608/aprh.2019.9839.1079
Esposito E, Cortesi R, Drechsler M, et al. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res. 2005;22:2163–2173. https://doi.org/10.1007/s11095-005-8176-x
Tomaszewska E, Soliwoda K, Kadziola K, et al. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticle colloids. J Nanomater. 2013;2013(1):e313081. https://doi.org/10.1155/2013/313081
Victorelli FD, Manni LS, Biffi S, et al. Potential of curcumin-loaded cubosomes for topical treatment of cervical cancer. J Colloid Interface Sci. 2022;620:419–430. https://doi.org/10.1016/j.jcis.2022.04.031
Mansour M, El Ezz TAA, Fattoh FN, AbouelFadl DM, Gad HA. Delineating the usage of dexamethasone-loaded cubosomes as a therapeutic armamentarium for hearing loss versus its protective effect: In vitro and in vivo animal study. J Drug Deliv Sci Technol. 2021;61:e102244. https://doi.org/10.1016/j.jddst.2020.102244
Prajapati V, Jain A, Jain R, Sahu S, Kohli DV. Treatment of cutaneous candidiasis through fluconazole-encapsulated cubosomes. Drug Deliv Transl Res. 2014;4:400–408. https://doi.org/10.1007/s13346-014-0202-2
Kurangi B, Jalalpure S, Jagwani S. Formulation and evaluation of resveratrol-loaded cubosomal nanoformulation for topical delivery. Curr Drug Deliv. 2021;18(5):607–619. https://doi.org/10.2174/1567201817666200902150646
Ali Z, Sharma PK, Warsi MH. Fabrication and evaluation of ketorolac-loaded cubosome for ocular drug delivery. J Appl Pharm Sci. 2016;6(9):204–208. https://doi.org/10.7324/JAPS.2016.60930
Elakkad YE, Younis MK, Allam RM, Mohsen AF, Khalil IA. Tenoxicam-loaded hyalcubosomes for osteoarthritis. Int J Pharm. 2021;601:e120483. https://doi.org/10.1016/j.ijpharm.2021.120483
Bessone CDV, Akhlaghi SP, Tártara LI, Quinteros DA, Loh W, Allemandi DA. Latanoprost-loaded phytantriol cubosomes for the treatment of glaucoma. Eur J Pharm Sci. 2021;160:e105748. https://doi.org/10.1016/j.ejps.2021.105748
Bei D, Zhang T, Murowchick JB, Youan B-BC. Formulation of dacarbazine-loaded cubosomes. part III. physicochemical characterization. AAPS PharmSciTech. 2010;11:1243–1249. https://doi.org/10.1208/s12249-010-9496-7
Nasr M, Younes H, Abdel-Rashid RS. Formulation and evaluation of cubosomes containing colchicine for transdermal delivery. Drug Deliv Transl Res. 2020;10:1302–1313. https://doi.org/10.1007/s13346-020-00785-6
Nasr M, Almawash S, Al Saqr A, Bazeed AY, Saber S, Elagamy HI. Bioavailability and antidiabetic activity of gliclazide-loaded cubosomal nanoparticles. Pharmaceuticals. 2021;14(8):e786. https://doi.org/10.3390/ph14080786
Khan S, Jain P, Jain S, Jain R, Bhargava S, Jain A. Topical delivery of erythromycin through cubosomes for acne. Pharm Nanotechnol. 2018;6(1):38–47. https://doi.org/10.2174/2211738506666180209100222
Al-Mahallawi AM, Abdelbary AA, El-Zahaby SA. Norfloxacin-loaded nano-cubosomes for enhanced management of otitis externa: In vitro and in vivo evaluation. Int J Pharm. 2021;600:e120490. https://doi.org/10.1016/j.ijpharm.2021.120490
Janakiraman K, Krishnaswami V, Sethuraman V, Rajendran V, Kandasamy R. Development of methotrexate-loaded cubosomes with improved skin permeation for the topical treatment of rheumatoid arthritis. Appl Nanosci. 2019;9:1781–1796. https://doi.org/10.1007/s13204-019-00976-9
Nasr M, Ghorab MK, Abdelazem A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm Sin B. 2015;5(1):79–88. https://doi.org/10.1016/j.apsb.2014.12.001
Zhai J, Tan FH, Luwor RB, et al. In vitro and in vivo toxicity and biodistribution of paclitaxel-loaded cubosomes as a drug delivery nanocarrier: a case study using an A431 skin cancer xenograft model. ACS Appl Bio Mater. 2020;3(7):4198–4207. https://doi.org/10.1021/acsabm.0c00269
Kulkarni CV, Vishwapathi VK, Quarshie A, et al. Self-assembled lipid cubic phase and cubosomes for the delivery of aspirin as a model drug. Langmuir. 2017;33(38):9907–9915. https://doi.org/10.1021/acs.langmuir.7b02486
Saber MM, Al-Mahallawi AM, Nassar NN, Stork B, Shouman SA. Targeting colorectal cancer cell metabolism through development of cisplatin and metformin nano-cubosomes. BMC Cancer. 2018;18:e822. https://doi.org/10.1186/s12885-018-4727-5
Salah S, Mahmoud AA, Kamel AO. Etodolac transdermal cubosomes for the treatment of rheumatoid arthritis: ex vivo permeation and in vivo pharmacokinetic studies. Drug Deliv. 2017;24(1):846–856. https://doi.org/10.1080/10717544.2017.1326539
Han S, Shen J-Q, Gan Y, et al. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol Sin. 2010;31:990–998. https://doi.org/10.1038/aps.2010.98
Mohsen AM, Younis MM, Salama A, Darwish AB. Cubosomes as a potential oral drug delivery system for enhancing the hepatoprotective effect of coenzyme Q10. J Pharm Sci. 2021;110(7):2677–2686. https://doi.org/10.1016/j.xphs.2021.02.007
Deruyver L, Rigaut C, Gomez-Perez A, Lambert P, Haut B, Goole J. In vitro evaluation of paliperidone palmitate-loaded cubosomes effective for nasal-to-brain delivery. Int J Nanomed. 2023;18:1085–1106. https://doi.org/10.2147/IJN.S397650
Patil SM, Sawant SS, Kunda NK. Inhalable bedaquiline-loaded cubosomes for the treatment of non-small cell lung cancer (NSCLC). Int J Pharm. 2021;607:e121046. https://doi.org/10.1016/j.ijpharm.2021.121046
Cytryniak A, Nazaruk E, Bilewicz R, et al. Lipidic cubic-phase nanoparticles (cubosomes) loaded with doxorubicin and labeled with 177Lu as a potential tool for combined chemo and internal radiotherapy for cancers. Nanomaterials. 2020;10(11):e2272. https://doi.org/10.3390/nano10112272
Yang Z, Peng X, Tan Y, et al. Optimization of the preparation process for an oral phytantriol-based amphotericin B cubosomes. J Nanomater. 2011;2011:1–10. https://doi.org/10.1155/2011/308016
Zaki RM, El Sayeh Abou El Ela A, Almurshedi AS, Aldosari BN, Aldossari AA, Ibrahim MA. Fabrication and assessment of orodispersible tablets loaded with cubosomes for the improved anticancer activity of simvastatin against the MDA-MB-231 breast cancer cell line. Polymers. 2023;15(7):e1774. https://doi.org/10.3390/polym15071774
Hundekar YR, Saboji J, Patil S, Nanjwade B. Preparation and evaluation of diclofenac sodium cubosomes for percutaneous administration. World J Pharm Pharm Sci. 2014;3(5):523–539.
Gaballa SA, El Garhy OH, Moharram H, Abdelkader H. Preparation and evaluation of cubosomes/cubosomal gels for ocular delivery of beclomethasone dipropionate for management of uveitis. Pharm Res. 2020;37:e198. https://doi.org/10.1007/s11095-020-02857-1
Nithya R, Jerold P, Siram K. Cubosomes of dapsone enhanced permeation across the skin. J Drug Deliv Sci Technol. 2018;48:75–81. https://doi.org/10.1016/j.jddst.2018.09.002
Hosny KM, Rizg WY, Alkhalidi HM, et al. Nanocubosomal-based in situ gel loaded with natamycin for ocular fungal diseases: development, optimization, in vitro, and in vivo assessment. Drug Deliv. 2021;28(1):1836–1848. https://doi.org/10.1080/10717544.2021.1965675
Eissa EM, Elkomy MH, Eid HM, et al. Intranasal delivery of granisetron to the brain via nanostructured cubosomes-based in situ gel for improved management of chemotherapy-induced emesis. Pharmaceutics. 2022;14(7):e1374. https://doi.org/10.3390/pharmaceutics14071374
Patil RP, Pawara DD, Gudewar CS, Tekade AR. Nanostructured cubosomes in an in situ nasal gel system: an alternative approach for the controlled delivery of donepezil HCl to the brain. J Liposome Res. 2019;29(3):264–273. https://doi.org/10.1080/08982104.2018.1552703
Mohsen AM, Salama AA, Asfour MH. Cubosome-based thermosensitive in situ gelling system for intranasal administration of lamotrigine with enhanced antiepileptic efficacy. Pharm Dev Technol. 2023;28(6):520–534. https://doi.org/10.1080/10837450.2023.2216755
Aboud HM, Hassan AH, Ali AA, Abdel-Razik A-RH. Novel in situ gelling vaginal sponges of sildenafil citrate-based cubosomes for uterine targeting. Drug Deliv. 2018;25(1):1328–1339. https://doi.org/10.1080/10717544.2018.1477858
Alhakamy NA, Hosny KM, Rizg WY, et al. Development and optimization of hyaluronic acid-poloxamer in-situ gel loaded with voriconazole cubosomes for enhancement of activity against ocular fungal infection. Gels. 2022;8(4):e241. https://doi.org/10.3390/gels8040241
Elgendy HA, Makky AM, Elakkad YE, Ismail RM, Younes NF. Syringeable atorvastatin-loaded eugenol-enriched PEGylated cubosomes in-situ gel for the intra-pocket treatment of periodontitis: statistical optimization and clinical assessment. Drug Deliv. 2023;30(1):e2162159. https://doi.org/10.1080/10717544.2022.2162159
Tekade A, Ghodke P, Patange A, Patil P. Nanostructured cubosomal in situ nasal gel for the treatment of migraine. J Drug Deliv Sci Technol. 2023;87:e104797. https://doi.org/10.1016/j.jddst.2023.104797
Morsi NM, Abdelbary GA, Ahmed MA. Silver sulfadiazine-based cubosome hydrogels for topical treatment of burns: development and in vitro/in vivo characterization. Eur J Pharm Biopharm. 2014;86(2):178–189. https://doi.org/10.1016/j.ejpb.2013.04.018
Murgia S, Bonacchi S, Falchi AM, et al. Drug-loaded fluorescent cubosomes: versatile nanoparticles for potential theranostic applications. Langmuir. 2013;29(22):6673–6679. https://doi.org/10.1021/la401047a
Abourehab MA, Ansari MJ, Singh A, et al. Cubosomes as an emerging platform for drug delivery: a review of the state of the art. J Mater Chem B. 2022;10(15):2781–2819. https://doi.org/10.1039/D2TB00031H
Almoshari Y. Development, therapeutic evaluation, and theranostic applications of cubosomes on cancers: an updated review. Pharmaceutics. 2022;14(3):e600. https://doi.org/10.3390/pharmaceutics14030600
Madheswaran T, Kandasamy M, Bose RJ, Karuppagounder V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov Today. 2019;24(7):1405–1412. https://doi.org/10.1016/j.drudis.2019.05.004
Tiberg F, Johnsson M. Drug delivery applications of non-lamellar liquid crystalline phases and nanoparticles. J Drug Deliv Sci Technol. 2011;21(1):101–109. https://doi.org/10.1016/S1773-2247(11)50009-7
Sen R, Gupta R, Singh S, Mantry S, Das S. A review on cubosome and virosome: the novel drug delivery system. UJPSR. 2017;3(1):24–33. https://doi.org/10.21276/UJPSR.2017.03.01.99
Zhai J, Fong C, Tran N, Drummond CJ. Non-lamellar lyotropic liquid crystalline lipid nanoparticles for the next generation of nanomedicine. ACS Nano. 2019;13(6):6178–6206. https://doi.org/10.1021/acsnano.8b07961
Boyd BJ. Characterization of drug release from cubosomes using the pressure ultrafiltration method. Int J Pharm. 2003;260(2):239–247. https://doi.org/10.1016/S0378-5173(03)00262-X
Rizwan S, Assmus D, Boehnke A, et al. Preparation of phytantriol cubosomes by solvent precursor dilution for the delivery of protein vaccines. Eur J Pharm Biopharm. 2011;79(1):15–22. https://doi.org/10.1016/j.ejpb.2010.12.034
Bhosale RR, Osmani RA, Harkare BR, Ghodake PP. Cubosomes: the inimitable nanoparticulate drug carriers. Scholars Acad J Pharm. 2013;2(6):481–486.
Copyright (c) 2025 Saman Ali, Nouman Farooq, Sabi Ur Rehman, Fazal Ur Rehman

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution (CC-BY) 4.0 License that allows others to share the work with an acknowledgement of the work’s authorship and initial publication in this journal









