Pharmacognostic, Phytochemical, and DNA Protection Assays of Eclipta alba, Ageratum conyzoides, Calendula officinalis, and Conyza bonariensis
Abstract
 Abstract Views: 0
Abstract Views: 0
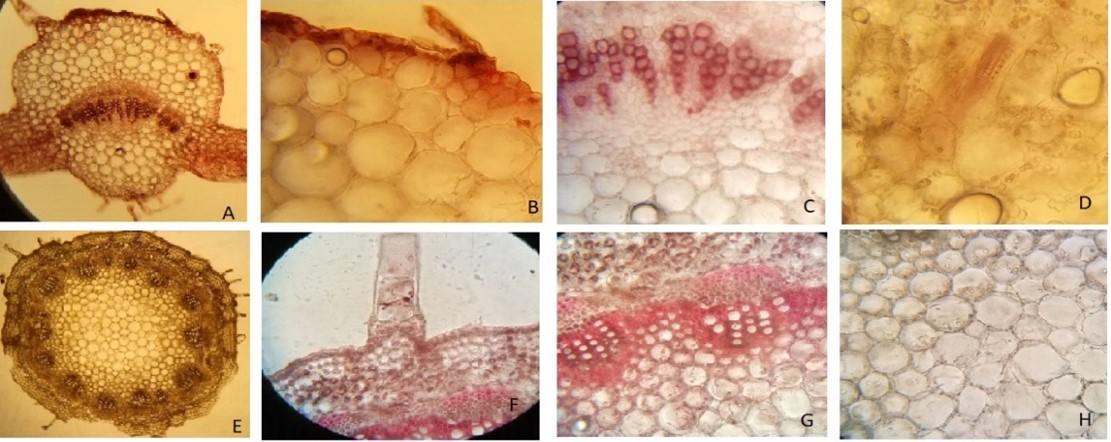
Asteraceae is the most famous family of ornamental flowering plants. Most of the plants included in this family have medicinal properties. The current research aimed to investigate the pharmacognostic and phytochemical standards of Eclipta alba L., Ageratum conyzoides L., Calendula officinalis L., and Conyza bonariensis L. to ensure their purity, safety, and efficacy as medicinal agents. The investigated parameters included pharmacognostic characterization, that is, powder microscopy, preliminary phytochemical analysis, physicochemical analysis, and in vitro DNA protection assay. The pharmacognostic characteristics evaluated via microscopy included the presence of fibers, linear covering trichomes, and glandular trichomes. Preliminary phytochemical screening confirmed the presence of different classes of phytoconstituents, such as glycosides, flavonoids, phenols, and triterpenoids. The DNA damage protection assay revealed that the crude methanolic extracts of these plants effectively protected human DNA from oxidative stress generated by free radicals of the oxidizing agent (Fenton’s reagent).
Downloads
References
Alamgir ANM. Phytoconstituents—active and inert constituents, metabolic pathways, chemistry and application of phytoconstituents, primary metabolic products, and bioactive compounds of primary metabolic origin. In: Therapeutic Use of Medicinal Plants and their Extracts: Volume 2: Phytochemistry and Bioactive Compounds. Springer; 2018:25–164. https://doi.org/10.1007/978-3-319-92387-1_2
Rehman SU, Arshad L, Saman A, Massey S, Khan S, Samad A. Unlocking the medicinal potential of Sarcococca saligna: green synthesis of silver and gold nanoparticles for enhanced antibacterial and antifungal applications. Pak J Agri Res. 2023;36(4):327–334. https://dx.doi.org/10.17582/journal.pjar/2023/36.4.327.334
Rehman SU, Anwar K, Saqib QNU, et al. In-Vitro antimicrobial analysis of aqueous methanolic extracts and crude saponins isolated from leaves and roots of Sarcococca saligna. Pak J Agri Res. 2019;32(2):268–274. http://dx.doi.org/10.17582/journal.pjar/2019/32.2.268.274
Gami B, Parabia M. Pharmacognostic evaluation of bark and seeds of Mimusops elengi L. Int J Pharm Pharm Sci. 2010;2(Suppl 4):110–113.
Kunle OF, Egharevba HO, Ahmadu PO. Standardization of herbal medicines-a review. Int J Biodivers Conserv. 2012;4(3):101–112. http://dx.doi.org/10.5897/IJBC11.163
Josa E, Barril G, Ruperto M. Potential effects of bioactive compounds of plant-based foods and medicinal plants in chronic kidney disease and dialysis: a systematic review. Nutrients. 2024;16(24):e4321. https://doi.org/10.3390/nu16244321
Ozsoy N, Can A, Yanardag R, Akev N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008;110(3):571–583. https://doi.org/10.1016/j.foodchem.2008.02.037
Zahra SA, Iqbal J, Abbasi BA, et al. Phylogenetic analysis of selected species of Asteraceae on the basis of RPS 11 Gene. Sci Rep. 2024;14:e24808. https://doi.org/10.1038/s41598-024-75991-0
Mueller MS, Karhagomba IB, Hirt HM, Wemakor E. The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: agricultural, chemical and clinical aspects. J Ethnopharmacol. 2000;73(3):487–493. https://doi.org/10.1016/S0378-8741(00)00289-0
Ain QT, Saleem N, Munawar N, Nawaz R, Naseer F, Ahmed S. Quest for malaria management using natural remedies. Front Pharmacol. 2024;15:e1359890. https://doi.org/10.3389/fphar.2024.1359890
Haddad PS, Azar GA, Groom S, Boivin M. Natural health products, modulation of immune function and prevention of chronic diseases. Evid Based Complement Altern Med. 2005;2(4):513–520. https://doi.org/10.1093/ecam/neh125
Kokate C, Purohit A, Gokhale S. Text Book of Pharmacognosy, Nirali Prakashan. Pragati Books Private Limited; 2008.
Inylieieva M, Karpiuk U. Determination of The Swelling Index for The Promising Plant Raw Materials as The Sources of Pectin Substances. Ministry of Health of Ukraine; 2023.
Aryan S, Mortazavian AM, Mohammadi F, Mahdavi V, Moazami N, Jazaeri S. Physicochemical properties of saponin containing Acanthophyllum laxiusculum extract: example application in foam stability and qualitative parameters for malt beverage industry. J Food Sci Technol. 2021;59:1577–1587. https://doi.org/10.1007/s13197-021-05169-3
Brain KR, Turner TD. The Practical Evaluation of Phytopharmaceuticals. Wright-Scientechnica; 1975.
Bhandary SK, Bhat VS, Sharmila K, Bekal MP. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. J Health Allied Sci NU. 2012;2(04):34–38. https://doi.org/10.1055/s-0040-1703609
Metscher BD. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 2009;9:e11. https://doi.org/10.1186/1472-6793-9-11
Alam F, Saqib QN. Pharmacognostic standardization and preliminary phytochemical studies of Gaultheria trichophylla. Pharm Biol. 2015;53(12):1711–1718. https://doi.org/10.3109/13880209.2014.1003355
Sevgi K, Tepe B, Sarikurkcu C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem Toxicol. 2015;77:12–21. https://doi.org/10.1016/j.fct.2014.12.006
Golla U, Bhimathati SSR. Evaluation of antioxidant and DNA damage protection activity of the hydroalcoholic extract of Desmostachya bipinnata L. Stapf. Sci World J. 2014;2014:e215084. https://doi.org/10.1155/2014/215084
Kala S, Johnson M, Janakiraman N, Arockiaraj AA, Raj SI, Bosco DBD. Pharmacognostic and phytochemical studies on some selected ethnomedicinal plants of Tamilnadu, South India. Int J Med Arom Plants. 2011;1(2):89–94.
Nivedithadevi D, Somasundaram R. Pharmacognostical and qualitative phytochemical Studies on the aerial parts of tephrosla purpurea (L). Int J Res Biol Sci. 2012;2(2):48–53.
Roopashree TS, Dang R. Standardization and phytochemical investigation of calendula officinalis, cassia Tora and Momordica Charantia seed extract. J Pharm Res. 2017;16(1):80–85. https://doi.org/10.18579/jpcrkc/2017/16/1/112481
Brewer M. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10(4):221–247. https://doi.org/10.1111/j.1541-4337.2011.00156.x
Donga S, Moteriya P, Pande J, Chanda S. Development of quality control parameters for the standardization of Pterocarpus santalinus Linn. F. leaf and stem. J Pharmacogn Phytochem. 2017;6(4):242–252.
Aschenbrenner A-K, Horakh S, Spring O. Linear glandular trichomes of Helianthus (Asteraceae): morphology, localization, metabolite activity and occurrence. AoB Plants. 2013;5:eplt028. https://doi.org/10.1093/aobpla/plt028
Copyright (c) 2025 Waheed Ullah Hafiz, Saiqa Ishtiaq, Sabi Ur Rehman, Laiba Arshad, Daud Ur Rehman, Hamid Saeed

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution (CC-BY) 4.0 License that allows others to share the work with an acknowledgement of the work’s authorship and initial publication in this journal









