Systematic Review of the SARS-CoV-2 Viral Vector Vaccine AstraZeneca (Azd1222)
Abstract
 Abstract Views: 1394
Abstract Views: 1394
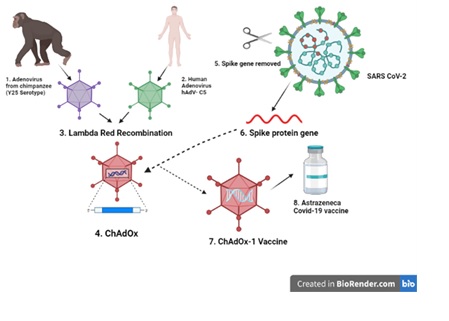
It has been more than two years since the spread of the COVID-19 pandemic all over the world. Scientists still are in search of a permanent treatment and cure of this infectious disease. In this regard, on 20th April, 2020, AstraZeneca in collaboration with the Oxford University came up with a recombinant adenovirus-based vaccine labeled as “ChAdOx1 nCoV-19” or “AZD1222”. Approximately, 22 viral vector-based vaccines are in the trial stage and ChAdOx1 nCoV-19 falls under the category of non-replicating viral vector-based vaccines. During vaccine development, ChAdOx-1 vector was specifically designed by red lambda recombination of Y25 serotype (chimpanzee) with HAdV-C5 serotype (human). In July 2020, clinical trials were initiated but due to some controversial side effects, these trials were halted for a while and resumed later on. AstraZeneca has been reported to generate both humoral and cell mediated immune responses. This vaccine exhibited 63% efficacy with few side effects. It also exhibited dwindling efficacy against the emerging variants of COVID-19. Since then, heterologous prime boost vaccination has been initiated, exhibiting elevated efficacy. Until now, 42.6% population of the world has been vaccinated with a single dose and this number is rising rapidly. However, due to the emerging variants of COVID-19, the efficacy of the majority of vaccines is decreasing. So, the manufacturers must work on making the vaccines more effective against these new variants as well. This review is written with the intention of summing up all the reported data regarding AZD1222 in order to provide a proper overview.
Downloads
References
Tahir MS, Salman Ullah M, Basit M, Zahid R. “COVID-19” and its Cardiovascular Complications-Review. Int J Sci Basic Appl Res. 2021;60(2):1–13.
Imam M, Zahid R, Muhammad RH, Abid S. Moderna (mRNA-1273) Covid-19 Vaccine, A Systematic Review. Int J Sci Basic Appl Res. 2021;60(2):87–99.
Krammer, F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516-527.
Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell Press. 2020;181(4):894-904. https://doi.org/10.1016/j.cell.2020.03.045
Padhi AK, Tripathi T. Can SARS-CoV-2 accumulate mutations in the S-protein to increase pathogenicity? ACS Pharmacology & Translational Science. 2020;3(5):1023-1026. https://doi.org/10.1021/acsptsci.0c00113
Wu SC. Progress and concept for COVID‐19 vaccine development. Biotechnol J. 2020;15(6):e2000147. https://dx.doi.org/10.1002%2Fbiot.202000147
Faruk Ö, Özge Ö. An update comprehensive review on the status of COVID-19: vaccines, drugs, variants and neurological symptoms. Turkish J Bio. 2021;45(SI4): 342-357. https://doi.org/10.3906/biy-2106-23
Basit M, Zahid, R, Saad Bin Tahir M, Riaz M. A Systematic Overview on SARS CoV-2 Pandemic & Pfizer-BioNTech COVID-19 Vaccine. Int J Innov Sci Res Technol. 2021;6(8):821–828.
World Health Organization. Background document on the AZD1222 vaccine against COVID-19 developed by Oxford University and AstraZeneca. World Health Organization; 2021. https://apps.who.int/iris/bitstream/handle/10665/339882/WHO-2019-nCoV-vaccines-SAGE-recommendation-AZD1222-background-2021.2-eng.pdf?sequence=1&isAllowed=y
Goff SP, Berg P. Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cells. Cell. 19756;9(4):695–705. https://doi.org/10.1016/0092-8674(76)90133-1
Xin-Yuan L, Huang WL, Qian QJ, et al. Cancer targeting gene-viro-therapy and ıts promising future: a trend in both cancer gene therapy and cancer virotherapy. In: Xin-Yuan L, Sidney P, Yu-Fang S, eds. Recent Advances in Cancer Research and Therapy. Elsevier Inc. 2012:33-83.
Ura T, Okuda K, Shimada M. Developments in viral vector-based vaccines. Vaccines. 2014;2(3):624-641. https://doi.org/10.3390/vaccines2030624
Morenweiser R. Downstream processing of viral vectors and vaccines. Gene Ther. 2005;12:S103-S110. https://doi.org/10.1038/sj.gt.3302624
Robbins PD, Steven CG. Viral vectors for gene therapy. Pharmacology & Therapeutics. 1998;80(1):35-47. https://doi.org/10.1016/S0163-7258(98)00020-5
Hansson M, ke Nygren P, Stahl S. Design and production of recombinant subunit vaccines. Biotechnol Appl Biochem. 2000;32(2):95-107. https://doi.org/10.1042/BA20000034
World Health Organization. COVID-19 vaccine tracker and landscape. World Health Organization;2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. npj Vaccines. 2021;6(1):1-17. https://doi.org/10.1038/s41541-021-00292-w
Guzmán-Martínez O, Guardado K, de Guevara EL, et al. IgG Antibodies Generation and Side Effects Caused by Ad5-nCoV Vaccine (CanSino Biologics) and BNT162b2 Vaccine (Pfizer/BioNTech) among Mexican Population. Vaccines. 2021;9(9):e999. https://doi.org/10.3390/vaccines9090999
Ghiasi NVR, Valizadeh R, Arabsorkhi M, et al. Efficacy and side effects of Sputnik V, Sinopharm and AstraZeneca vaccines to stop COVID-19; a review and discussion. Immunopathologia Persa. 2021;7(2):e31. https://doi.org/10.34172/ipp.2021.31
Zare H, Rezapour H, Mahmoodzadeh S, Fereidouni M. Prevalence of COVID-19 vaccines (Sputnik V, AZD-1222, and Covaxin) side effects among healthcare workers in the Birjand, Iran. Int. Immunopharmacology. 2021;101:e108351. https://doi.org/10.1016/j.intimp.2021.108351
Lukashev AN, Zamyatnin AA. Viral vectors for gene therapy: current state and clinical perspectives. Biochem. 2016;81(7):700-708. https://doi.org/10.1134/S0006297916070063
Kang J, Ismail AM, Dehghan S, et al. (2020). Genomics‐based re‐examination of the taxonomy and phylogeny of human and simian Mastadenoviruses: an evolving whole genomes approach, revealing putative zoonosis, anthroponosis, and amphizoonosis. Cladistics. 2020;36(4):358-373. https://doi.org/10.1111/cla.12422
Saleh NA, Elhaes H, Ibrahim M. Design and development of some viral protease inhibitors by QSAR and molecular modeling studies. In: Gupta SP, eds. Viral proteases and their inhibitors. Academic Press; 2017:25–58. https://doi.org/10.1016/B978-0-12-809712-0.00002-2
Feng L, Wang Q, Shan C, et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat Commun. 2020;11(1):1-11. https://doi.org/10.1038/s41467-020-18077-5
Garofalo M, Staniszewska M, Salmaso S, et al. Prospects of replication-deficient adenovirus based vaccine development against SARS-CoV-2. Vaccines. 2020;8(2):e293. https://doi.org/10.3390/vaccines8020293
Mendonça SA, Lorincz R, Boucher P, Curiel DT. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. npj Vaccines. 2021;6(1):1-14 https://doi.org/10.1038/s41541-021-00356-x
Singh, S., Kumar, R., & Agrawal, B. Adenoviral vector-based vaccines and gene therapies: Current status and future prospects. In: Desheva Y, ed. Adenoviruses. BoD-Books;2019:53-91. http://dxdoi.org/10.5772/intechopen.79697
Cottingham MG, Carroll F, Morris SJ, et al. Preventing spontaneous genetic rearrangements in the transgene cassettes of adenovirus vectors. Biotechnol Bioeng. 2012;109(3):719-728. https://doi.org/10.1002/bit.24342
Morris SJ, Sebastian S, Spencer AJ, Gilbert SC. Simian adenoviruses as vaccine vectors. Future Virol. 2016;11(9):649-659. https://doi.org/10.2217/fvl-2016-0070
Dicks MD, Spencer AJ, Edwards NJ, et al. (2012). A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PloS One. 2012;7(7):e40385. https://doi.org/10.1371/journal.pone.0040385
Committee for Medicinal Products for Human Use. COVID-19 Vaccine AstraZeneca. CMPH. Accessed January 29, 2021. https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-astrazeneca-product-information-approved-chmp-29-january-2021-pending-endorsement_en.pdf
Corum J, Zimmer C. How the Oxford-AstraZeneca Vaccine Works. The New York Times. May 7, 2021. https://www.nytimes.com/interactive/2020/health/oxford-astrazeneca-covid-19-vaccine.html
Theodorou DJ, Theodorou SJ, Axiotis A, Gianniki M, Tsifetaki N. COVID-19 vaccine-related myositis. Int J Med. 2021;14(6);424-425. https://doi.org/10.1093/qjmed/hcab043
Tosi MF. Innate immune responses to infection. J Allergy Clinic Immunol. 2005;116(2):241-249. https://doi.org/10.1016/j.jaci.2005.05.036
Quast I, Tarlinton D. B cell memory: Understanding COVID-19. Immunity. 2021;54(2):205-210. https://doi.org/10.1016/j.immuni.2021.01.014
Röltgen K, Boyd SD. Antibody and B cell responses to SARS-CoV-2 infection and vaccination. Cell Host Microbe. 2021;27(7):1063-1075. https://doi.org/10.1016/j.chom.2021.06.009
Schenten D, Medzhitov R. Chapter 3- The control of adaptive immune responses by the innate immune system. Advances Immunol. 2011;109:87-124. https://doi.org/10.1016/B978-0-12-387664-5.00003-0
Nasdaq. AZN, IQV team up to accelerate COVID-19 vaccine work, RIGL’s ITP drug repurposed, IMV on watch. RTTNews. July 15, 2020. https://www.nasdaq.com/articles/azn-iqv-team-up-to-accelerate-covid-19-vaccine-work-rigls-itp-drug-repurposed-imv-on-watch
Wu KJ, Thomas K. AstraZeneca pauses vaccine trial for safety review. The New York Times. Accessed September 9, 2020. https://www.nytimes.com/2020/09/08/health/coronavirus-astrazeneca-vaccine-safety.html
Loftus P. AstraZeneca Covid-19 vaccine trials resume in U.K. The Wall Street Journal. Accessed February 20, 2021. https://www.wsj.com/articles/astrazeneca-covid-19-vaccine-trials-resume-in-the-u-k-11599922981
Callaway E. Why Oxford's positive COVID vaccine results are puzzling scientists. Nature. 2020;588:16–18. https://doi.org/10.1038/d41586-020-03326-w
Roberts M. Oxford/AstraZeneca Covid vaccine “dose error” explained. BBC News. 2020. https://www.bbc.com/news/health-55086927
Robbins R, Mueller B. After admitting mistake, AstraZeneca faces difficult questions about ıts vaccine. The New York Times. Accessed March 27, 2021. https://www.bbc.com/news/health-55086927
Voysey M, Clemens SAC, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. https://doi.org/10.1016/S0140-6736(21)00432-3
Robbins R, Kaplan S. AstraZeneca vaccine trial results are questioned by U.S. health officials. The New York Times. Accessed March 23, 2021. https://www.nytimes.com/2021/03/23/business/astrazeneca-vaccine-questions.html
University of Oxford. University of Oxford to study nasal administration of COVID-19 vaccine. University of Oxford. 2021. https://www.ox.ac.uk/news/2021-03-25-university-oxford-study-nasal-administration-covid-19-vaccine
Department of Health and Social Care. Oxford University/AstraZeneca vaccine authorized by UK medicines regulator. Government of UK. 2020. https://www.gov.uk/government/news/oxford-universityastrazeneca-vaccine-authorised-by-uk-medicines
European Medicines Agency. Vaxzevria (previously COVID-19 Vaccine AstraZeneca) Share RSS. EMA. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca
Wikipedia. List of COVID-19 vaccine authorizations. (n.d.). https://en.wikipedia.org/wiki/List_of_COVID-19_vaccine_authorizations#Oxford%E2%80%93AstraZeneca
Coronavirus (COVID-19) Vaccinations. Our World in Data. Covid Vaccines. Accessed September, 2021. https://ourworldindata.org/covid-vaccinations
Khadija H, Zahid R, Gulzar S, Ahmad M. Emerging Variants of SARS Cov-2 “A New Challenge of 2021 for the World”. Arch Microbiol Immunol. 2021;5(4):373-383 https://doi.org/10.26502/ami.93650070
Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. https://doi.org/10.1038/s41591-021-01377-8
Krause P, Fleming TR, Longini I, et al. COVID-19 vaccine trials should seek worthwhile efficacy. Lancet. 2020;396(10253):741–743. https://doi.org/10.1016/S0140-6736(20)31821-3
Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461-2462. https://doi.org/10.1016/S0140-6736(21)01358-1
Lu S. Heterologous prime–boost vaccination. Curr Opin Immunol. 2009;21(3):346-351. https://doi.org/10.1016/j.coi.2009.05.016
Sample I, Grover N. Mixing Covid vaccines offers stong immune protection. The Guardian. Accessed June 28, 2021. https://www.theguardian.com/world/2021/jun/28/astrazeneca-vaccine-protection-uncompromised-by-gap-over-12-weeks-study
Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27:1530-1535. https://doi.org/10.1038/s41591-021-01464-w
Wall EC, Wu M, Harvey R, et al. AZD1222-induced neutralising antibody activity against SARS-CoV-2 Delta VOC. Lancet. 2021;398(10296):207-209. https://doi.org/10.1016/S0140-6736(21)01462-8
Copyright (c) 2022 rohama zahid , Samreen Riaz

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.









