Synthesis, Structural Elucidation, and Biological Potential of Novel Sulfonamide Drugs
Abstract
 Abstract Views: 110
Abstract Views: 110
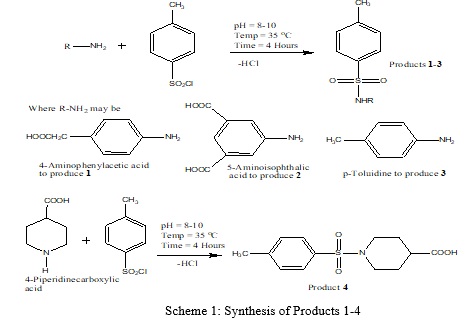
Sulfonamides are anti-microbial and anti-inflammatory agents used widely for the treatment of infections. The current research dealt with the synthesis of four sulfonamides formed by the reaction of p-toluenesulfonyl chloride with 4-amino phenyl acetic acid, 5-amino isophthalic acid, 4-piperidine carboxylic acid, and toluidine. The respective structures of sulfonamide drugs were verified by elemental analysis (CHNS), FT-IR spectroscopy, and thermogravimetry. The elemental analysis (CHNS) data conformed to the proposed chemical composition of the products 1-4. The FT-IR spectra verified the formation of sulfonamide drugs. Thermogravimetric analysis (TGA) of data in the range of 25-800oC showed that the evolved components and residues were in good agreement with the molecular skeletons of the products. The antibacterial potential of sulfonamide drugs was evaluated against bacterial strains using the disc diffusion method. The zones of inhibition were found to be larger against Escherichia coli as compared to Bacillus Subtilis. Cytotoxicity of products was in the acceptable range of 0.3-3.1%, as compared to triton X100. It indicates the safety of these products as future medicinal drugs for human beings.
Downloads
References
Mondal S, Malakar S. Synthesis of sulfonamide and their synthetic and therapeutic applications: Recent advances. Tetrahedron. 2020;76(48):e131662. https://doi.org/10.1016/j.tet.2020.131662
Tačić A, Nikolić V, Nikolić L, Savić I. Antimicrobial sulfonamide drugs. Adv Technol. 2017;6(1):58-71.
Giles A, Foushee J, Lantz E, Gumina G. Sulfonamide allergies. Phar. 2019;7(3):e132.
Wilden JD. The sulfonamide motif as a synthetic tool. J Chem Res. 2010;34(10):541- 548. https://doi.org/10.3184/030823410X12857514635822
Gulçin İ, Taslimi P. Sulfonamide inhibitors: a patent review 2013-present. Expert Opin Ther Pat. 2018;28(7):541-549. https://doi.org/10.1080/13543776.2018.1487400
Zhong Z, Ji X, Xing R, et al. The preparation and antioxidant activity of the sulfanilamide derivatives of chitosan and chitosan sulfates. Bioorg Med Chem. 2007;15(11):3775-3782. https://doi.org/10.1016/j.bmc.2007.03.036
Apaydın S, Török M. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorganic Med Chem Lett. 2019;29(16):2042-2050. https://doi.org/10.1016/j.bmcl.2019.06.041
Al-Rashida M, Hussain S, Hamayoun M, Altaf A, Iqbal J. Sulfa drugs as inhibitors of carbonic anhydrase: new targets for the old drugs. Biomed Res Int. 2014;2014:e162928. https://doi.org/10.1155/2014/162928
Kołaczek A, Fusiarz I, Ławecka J, Branowska D. Biological activity and synthesis of sulfonamide derivatives: a brief review. Chemik. 2014;68(7):620-628.
Alsughayer A, Elassar A-ZA, Mustafa S, Al Sagheer F. Synthesis, structure analysis and antibacterial activity of new potent sulfonamide derivatives. J Biomater Nanobiotechnol. 2011;2(2):144-149. https://doi.org/10.4236/jbnb.2011.22018
Cohen HN, Beastall G, Ratcliffe W, Gray C, Watson I, Thomson J. Effects on human thyroid function of sulphonamide and trimethoprim combination drugs. Br Med J. 1980;281(6241):646-647. https://doi.org/10.1136/bmj.281.6241.646
Comby F, Lagorce J, Moulard T, Buxeraud J, Raby C. Antibacterial sulfonamides, antiparasitic and antifungal derivatives of imidazole: evaluation of their antithyroid effects in the rat. Vet Res. 1993;24(4):316-326.
Domagk G. Ein beitrag zur chemotherapie der bakteriellen infektionen. DMW-Deutsche Med Wochenschr. 1935;61(07):250-253. https://doi.org/10.1055/s-0028-1129486
Azevedo-Barbosa H, Dias DF, Franco LL, Hawkes JA, Carvalho DT. From antibacterial to antitumour agents: a brief review on the chemical and medicinal aspects of sulfonamides. Mini Rev Med Chem. 2020;20(19):2052-2066. https://doi.org/10.2174/1389557520666200905125738
Mondal S. Sulfonamide synthesis under green conditions. Synth Commun. 2021;51(7):1023-1044. https://doi.org/10.1080/00397911.2020.1870238
Strom BL, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349(17):1628-1635. https://doi.org/10.1056/NEJMoa022963
Khan DA, Knowles SR, Shear NH. Sulfonamide hypersensitivity: Fact and fiction. J Allergy Clin Immunol: In Pract. 2019;7(7):2116-2123. https://doi.org/10.1016/j.jaip.2019.05.034
Razavi M, Marathe NP, Gillings MR, Flach C-F, Kristiansson E, Joakim Larsson D. Discovery of the fourth mobile sulfonamide resistance gene. Microbiome. 2017;5(1):1-12. https://doi.org/10.1186/s40168-017-0379-y
Ji L, Chen W, Zheng S, Xu Z, Zhu D. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir. 2009;25(19):11608-11613. https://doi.org/10.1021/la9015838
García-Galán MJ, Díaz-Cruz MS, Barceló D. Identification and determination of metabolites and degradation products of sulfonamide antibiotics. TrAC-Trend Anal Chem. 2008;27(11):1008-1022. https://doi.org/10.1016/j.trac.2008.10.001
Accinelli C, Koskinen WC, Becker JM, Sadowsky MJ. Environmental fate of two sulfonamide antimicrobial agents in soil. J Agric Food Chem. 2007;55(7):2677-2682. https://doi.org/10.1021/jf063709j
Gutiérrez IR, Watanabe N, Harter T, Glaser B, Radke M. Effect of sulfonamide antibiotics on microbial diversity and activity in a Californian Mollic Haploxeralf. J Soils Sediments. 2010;10(3):537-544. https://doi.org/10.1007/s11368-009-0168-8
Chen J, Xie S. Overview of sulfonamide biodegradation and the relevant pathways and microorganisms. Sci Total Environ. 2018;640:1465-1477. https://doi.org/10.1016/j.scitotenv.2018.06.016
Verma SK, Verma R, Xue F, Thakur PK, Girish Y, Rakesh K. Antibacterial activities of sulfonyl or sulfonamide containing heterocyclic derivatives and its structure-activity relationships (SAR) studies: A critical review. Bioorg Med Chem. 2020;105:e104400. https://doi.org/10.1016/j.bioorg.2020.104400
Sadarangani SP, Estes LL, Steckelberg JM. Non–anti-infective effects of antimicrobials and their clinical applications: A review. Elsevier; 2015. https://doi.org/10.1016/j.mayocp.2014.09.006
Prescott JF. Sulfonamides, diaminopyrimidines, and their combinations. Antimicrob Ther Veter Med. 2013;5:279-294. https://doi.org/10.1002/9781118675014.ch17
Fritsche TR, McDermott PF, Shryock TR, Walker RD. Agar dilution and disk diffusion susceptibility testing of Campylobacter spp. J Cli Microbiol. 2007;45(8):2758-2759. https://doi.org/10.1128/JCM.00569-07
Hussain S, Ali S, Shahzadi S, Rizzoli C, Shahid M. Diorganotin (IV) complexes with monohydrate disodium salt of iminodiacetic acid: synthesis, characterization, crystal structure and biological activities. J Chin Chem Soc. 2015;62(9):793-802. https://doi.org/10.1002/jccs.201500274
Hussain S, Ali S, Shahzadi S, Tahir MN, Shahid M. Synthesis, characterization, biological activities, crystal structure and DNA binding of organotin (IV) 5- chlorosalicylates. J Coord Chem. 2015;68(14):2369-2387.
https://doi.org/10.1080/00958972.2015.1046849
Hussain S, Ali S, Shahzadi S, et al. Organotin (IV) complexes with 5-aminoisophthalic acid: Synthesis, characterization, theoretical study, and biological activities. Russ J Gen Chem. 2015;85(10):2386-2394. https://doi.org/10.1134/S1070363215100266
Fatima J, Hussain S, Ali S, Shahzadi S, Ramzan S, Shahid M. Organo Sn (IV) and Pd (II) complexes with various oxygen and sulphur donor ligands: Synthesis, spectroscopy and biological activity. Russ J Gen Chem. 2016;86(12):2914-2923. https://doi.org/10.1134/S1070363216120604
Sharma P, Sharma JD. In vitro hemolysis of human erythrocytes—by plant extracts with antiplasmodial activity. J Ethnopharmacol. 2001;74(3):239-243. https://doi.org/10.1016/S0378-8741(00)00370-6
Hussain S, Ali S, Shahzadi S, Sharma SK, Qanungo K, Shahid M. Synthesis, characterization, semiempirical and biological activities of organotin (IV) carboxylates with 4-piperidinecarboxylic acid. Bioinorg Chem Appl. 2014;2014:e959203. https://doi.org/10.1155/2014/959203
Balas V, Verginadis I, Geromichalos G, et al. Synthesis, structural characterization and biological studies of the triphenyltin (IV) complex with 2-thiobarbituric acid. Eur J Med Chem. 2011;46(7):2835-2844. https://doi.org/10.1016/j.ejmech.2011.04.005
Hussain S, Bukhari IH, Ali S, Shahzadi S, Shahid M, Munawar KS. Synthesis and spectroscopic and thermogravimetric characterization of heterobimetallic complexes with Sn (IV) and Pd (II); DNA binding, alkaline phosphatase inhibition and biological activity studies. J Coord Chem. 2015;68(4):662-677. https://doi.org/10.1080/00958972.2014.994515
Hussain S, Ali S, Shahzadi S, Shahid M. Heterobimetallic complexes containing Sn (IV) and Pd (II) with 4-(2-Hydroxyethyl) piperazine-1-carbodithioic acid: Synthesis, characterization and biological activities. Cogent Chem. 2015;1(1):e1029038. https://doi.org/10.1080/23312009.2015.1029038
Hussain S, Ali S, Shahzadi S, Sharma SK, Qanungo K, Bukhari IH. Homobimetallic complexes containing Sn (IV) with acetylene dicarboxylic acid: their syntheses and structural interpretation by spectroscopic, semi-empirical, and DFT techniques. J Coord Chem. 2012;65(2):278-285. https://doi.org/10.1080/00958972.2011.648186
Rehman H, Qadir A, Ali Z, Nazir S, Zahra A, Shahzady TG. Synthesis and characterization of novel sulfonamides derivatives and their antimicrobial, antioxidant and cytotoxicity evaluation. Bull Chem Soc Ethiop. 2017;31(3):491-498. https://doi.org/10.4314/bcse.v31i3.13
Khan FA, Mushtaq S, Naz S, et al. Sulfonamides as potential bioactive scaffolds. Curr Org Chem. 2018;22(8):818-830. https://doi.org/10.2174/1385272822666180122153839
Ovung A, Bhattacharyya J. Sulfonamide drugs: Structure, antibacterial property, toxicity, and biophysical interactions. Biophys Rev. 2021;13(2):259-272. https://doi.org/10.1007/s12551-021-00795-9
Idrees S, Sarfraz R, Riaz M, et al. Evaluation of antidiabetic, antioxidant, antimicrobial and cytotoxicity properties of avena sativa roots extracts. Oxid Commun. 2017;40(2):613-623.
Copyright (c) 2022 Shabbir Hussain, Shakila Riaz, Hajira Rehman, Muhammad Shahid, Syed Mustansar Abbas

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










