Combined Effect of Honey, Neem (Azadirachta Indica), and Turmeric against Staphylococcus Aureus and E. Coli Isolated from a Clinical Wound Sample
Abstract
 Abstract Views: 241
Abstract Views: 241
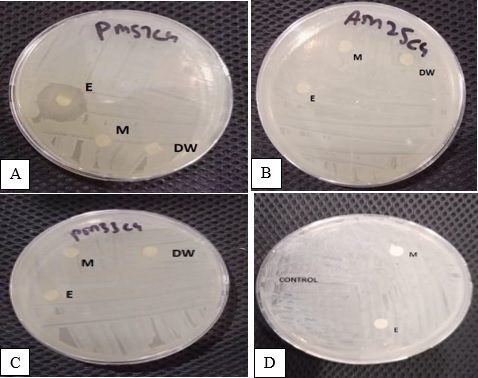
Antimicrobial resistance has become evident all over the world. Resistance to antibiotics has become a concern in case of a wide variety of bacterial species, both pathogenic and commensal. More recently, E. coli, pseudomonas, Staphylococcus aureus, Streptococcus, and Enterococci were found to be adversely affecting the healthcare structures of the world, particularly where acute and long-term care facilities are available. Microbial species were identified by Vitek compact-2 and MALDI-TOF, while antibiogram sensitivity was checked using Kirby Bauer disc diffusion and well diffusion method. The study used 20 wound samples from five (5) men and fifteen (15) women. Thirty-four (34) purified colonies of bacteria were created, in which 8 were E. coli and 2 were S. aureus. The effects of neem, turmeric, and honey with ethanol extracts showed the maximum zone of inhibition against clinically isolated E. coli, such as PM33C4 and AM25C4. While, methanol extract also showed the maximum zone of inhibition against PM56C4, AF34C4, and PM57C4, using disc diffusion and well diffusion methods. Correspondingly, the effect of neem, turmeric, and honey with ethanol extracts showed maximum inhibition against S. aureus. Whereas, methanol extract showed a sensitive zone of inhibition only against PM54C1 using the disc diffusion method. Hence, it was determined that natural ingredients such as honey, turmeric, and neem are an effective alternative to antibiotics because they manifest excellent antimicrobial activity against clinical bacterial isolates.
Downloads
References
Sisay M, Worku T, Edessa D. Microbial epidemiology and antimicrobial resistance patterns of wound infection in Ethiopia: a meta-analysis of laboratory-based cross-sectional studies. BMC Pharmacol Toxicol. 2019;20(1):1-19. https://doi.org/10.1186/s40360-019-0315-9
Tom HAM. Prevalence of hospital aquired infection in postoperative wound and antibiotic resistance at kassala teaching hospital, KassalaStata, Sudan (2014–2016)[master’s thesis]. Sudan: University of Gezira; 2016. http://repo.uofg.edu.sd/handle/123456789/1252
Wadi MA. Antibacterial activity of different global honey samples against standard organisms. Asian J. Microbiol Biotech Env Sci. 2019;21(4):924-930.
Kanimozhi S, Kathiresan G, Kathalingam A, Kim H-S, Doss M. Organic nanocomposite Band-Aid for chronic wound healing: A novel honey-based nanofibrous scaffold. Appl Nanosci. 2020;10(5):1639-1652. https://doi.org/10.1007/s13204-019-01247-3
Tripathy R, Nair SV, Lakshman V, Raj S, Otta SP. Wound-healing potential of Nimbadi Kalka in diabetic foot ulcer: a clinical study. J Ind Sys Med. 2020;8(2):102. https://doi.org/10.4103/JISM.JISM_59_20
Tariq F, Ahmed N, Afzal M, Khan MAU, Zeshan B. Synthesis, characterization and antimicrobial activity of bacillus subtilis-derived silver Nanoparticles against multidrug-resistant bacteria. Jundishapur J Microbiol. 2020;13(5): e91934. https://doi.org/10.5812/jjm.91934
Shaikh BT, Hatcher J. Complementary and alternative medicine in Pakistan: prospects and limitations. Evid Based Complementary Altern Med. 2005;2(2):139-142. https://doi.org/10.1093/ecam/neh088
Ahmed N, Zeshan B, Naveed M, Afzal M, Mohamed M. Antibiotic resistance profile in relation to virulence genes fimH, hlyA and usp of uropathogenic E. coli isolates in Lahore, Pakistan. Trop Biomed. 2019;36(2):559-68.
Kali A, Devaraj Bhuvaneshwar P, Charles M, Seetha KS. China supply custom reclaimed timber shelves suppliers & manufacturers Quotes-INNOVATIVE. J Basic Clinic Pharm. 2016;7(3):93.
Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica). Curr Sci. 2002:82(11):1336-1345.
Halawani E, Shohayeb M. Survey of the antibacterial activity of Saudi and some international honeys. J Microbiol Antimicro. 2011;3(4):94-101. https://doi.org/10.5897/JMA2021.0449
Kaškonienė V, Venskutonis P, Čeksterytė V. Carbohydrate composition and electrical conductivity of different origin honeys from Lithuania. LWT-Food Sci Technol. 2010;43(5):801-817. https://doi.org/10.1016/j.lwt.2010.01.007
Zafar M, Latafat T, Zehra A, Farooqui Y. Therapeutic properties of honey: A review of literature. Res Rev A J Pharmacol. 2020;10:41-49.
Singh V, Roy M, Garg N, Kumar A, Arora S, Malik DS. An Insight into the Dermatological Applications of Neem: A Review on Traditional and Modern Aspect. Recent Advan Anti-Infective Drug Discov Forme Recent Patents Anti-Infect Drug Discovery. 2021;16(2):94-121. https://doi.org/10.2174/2772434416666210604105251
Zapata A, Ramirez-Arcos S. A comparative study of McFarland turbidity standards and the Densimat photometer to determine bacterial cell density. Curr Microbiol. 2015;70(6):907-909. https://doi.org/10.1007/s00284-015-0801-2
Akintobi O, Onoh C, Ogele J, Idowu A, Ojo O, Okonko I. Antimicrobial activity of Zingiber officinale (ginger) extract against some selected pathogenic bacteria. Nat Sci. 2013;11(1):7-15.
El-Hadedy D, El-Nour SA. Identification of Staphylococcus aureus and Escherichia coli isolated from Egyptian food by conventional and molecular methods. J Genet Eng Biotechnol. 2012;10(1):129-35. https://doi.org/10.1016/j.joim.2018.07.005
Tanih NF, Sekwadi E, Ndip RN, Bessong PO. Detection of pathogenic Escherichia coli and Staphylococcus aureus from cattle and pigs slaughtered in abattoirs in Vhembe district, South Africa. Sci World J. 2015;2015:e195972 https://doi.org/10.1155/2015/195972
Becerra SC, Roy DC, Sanchez CJ, Christy RJ, Burmeister DM. An optimized staining technique for the detection of Gram positive and Gram negative bacteria within tissue. BMC Res Notes. 2016;9(1):1-10. https://doi.org/10.1186/s13104-016-1902-0
Saadi Al-Baer A, Hussein AA. Isolation and identification of escherichia coli producing cytosine deaminase from Iraqi patients. Int J Adv Res Biol Sci. 2017;4(11):1-6. http://dx.doi.org/10.22192/ijarbs.2017.04.11.001
Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the infectious diseases society of America. Clin Infect Dis. 2008;46(2):155-164. https://doi.org/10.1086/524891
Walker B, Barrett S, Polasky S, et al. Looming global-scale failures and missing institutions. Science. 2009;325(5946):1345-1346. https://doi/org/10.1126/science.1175325
Bantawa K, Sah SN, Limbu DS, Subba P, Ghimire A. Antibiotic resistance patterns of Staphylococcus aureus, Escherichia coli, Salmonella, Shigella and Vibrio isolated from chicken, pork, buffalo and goat meat in eastern Nepal. BMC Research Notes. 2019;12(1):1-6. https://doi.org/10.1186/s13104-019-4798-7
Mahon CR, Lehman DC, Manuselis G. Textbook of diagnostic microbiology-e-book. Elsevier Health Sciences; 2018. https://doi.org/10.5812/iji.58844
Österblad M, Hakanen A, Manninen R, et al. A between-species comparison of antimicrobial resistance in enterobacteria in fecal flora. Antimicrob Agents Chemother. 2000;44(6):1479-1484. https://doi.org/10.1128/AAC.44.6.1479-1484.2000
Kurutepe S, Surucuoglu S, Sezgin C, Gazi H, Gulay M, Ozbakkaloglu B. Increasing antimicrobial resistance in Escherichia coli isolates from community-acquired urinary tract infections during 1998-2003 in Manisa, Turkey. Jpn J Infect Dis. 2005;58(3):159-161. https://doi.org/10.1111/j.1469-0691.2004.01057.x
Gurung RR, Maharjan P, Chhetri GG. Antibiotic resistance pattern of Staphylococcus aureus with reference to MRSA isolates from pediatric patients. Future Sci OA. 2020;6(4):eFSO464. https://doi.org/10.2144/fsoa-2019-0122
Pandey S, Raza M, Bhatta C. Prevalence and antibiotic sensitivity pattern of methicillin-resistant-Staphylococcus aureus in Kathmandu Medical College-Teaching Hospital. J Institute Med Nepal. 2012;34(1):13-17. https://doi.org/10.3126/jiom.v34i1.9117
Mandal S, DebMandal M, Pal NK, Saha K. Antibacterial activity of honey against clinical isolates of Escherichia coli, Pseudomonas aeruginosa and Salmonella enterica serovar Typhi. Asian Pac J Trop Med. 2010;3(12):961-964. https://doi.org/10.1016/S1995-7645(11)60009-6
Mulu A, Tessema B, Derbie F. In vitro assessment of the antimicrobial potential of honey on common human pathogens. Ethiopian J Health Develop. 2004;18(2):107-112.
French VM, Cooper RA, Molan PC. The antibacterial activity of honey against coagulase-negative staphylococci. J Antimicrob Chemoth. 2005;56(1):228-231. https://doi.org/10.1093/jac/dki193
Atrott J, Henle T. Methylglyoxal in manuka honey—correlation with antibacterial properties. Czech J Food Sci. 2009;27(Special Issue):S163-S5. https://doi.org/10.17221/911-CJFS
Maghsoudi H, Salehi F, Khosrowshahi M, Baghaei M, Nasirzadeh M, Shams R. Comparison between topical honey and mafenide acetate in treatment of burn wounds. Ann Burn Fire Disasters. 2011;24(3):132-137.
Afrose R, Saha S, Banu L, et al. Antibacterial effect of curcuma longa (Turmeric) against staphylococcus aureus and Escherichia coli. Mymensingh Med J. 2015;24(3):506-515.
Gul P, Bakht J. Antimicrobial activity of turmeric extract and its potential use in food industry. J Food Sci Technol. 2015;52(4):2272-2279. https://doi.org/10.1007/s13197-013-1195-4
Lim HS, Park SH, Ghafoor K, Hwang SY, Park J. Quality and antioxidant properties of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. Food Chem. 2011;124(4):1577-1582. https://doi.org/10.1016/j.foodchem.2010.08.016
Pezeshk S, Rezaei M, Hosseini H. Effects of turmeric, shallot extracts, and their combination on quality characteristics of vacuum‐packaged rainbow trout stored at 4±1 C. J Food Sci. 2011;76(6):M387-M91. https://doi.org/10.1111/j.1750-3841.2011.02242.x
Prakash D, Suri S, Upadhyay G, Singh BN. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int J Food Sci Nutr. 2007;58(1):18-28. https://doi.org/10.1080/09637480601093269
Kim KJ, Yu HH, Cha JD, Seo SJ, Choi NY, You YO. Antibacterial activity of Curcuma longa L. against methicillin‐resistant Staphylococcus aureus. Phytother Res. 2005;19(7):599-604. https://doi.org/10.1002/ptr.1660
Francine U, Jeannette U, Pierre RJ. Assessment of antibacterial activity of neem plant (Azadirachta indica) on Staphylococcus aureus and Escherichia coli. J Med Plants Stud. 2015;3(4):85-91.
Maleki L, Sadeghian-Rizi T, Ghannadian M, Sanati MH, Shafizadegan S, Sadeghi-Aliabadi H. Antibacterial activity of Azadirachta indica leaf extracts against some pathogenic standards and clinical bacterial isolates. Avicenna J Clin Microbiol Infect. 2017;5(1):e12987- https://doi.org/10.5812/ajcmi.12987
Banna QR, Parveen F, Iqbal MJ. Growth inhibitory effect of ethanolic neem leaves extract on Klebsiella, Salmonella and Staphylococcus aureus. Bangladesh J Pharmacol. 2014;9(3):347-350. https://doi.org/10.3329/bjp.v9i3.19454
Arora N, Koul A, Bansal M. Chemopreventive activity of Azadirachta indica on two‐stage skin carcinogenesis in murine model. Phytother Res. 2011;25(3):408-416. https://doi.org/10.1002/ptr.3280
Ghimeray AK, Jin C-W, Ghimire BK, Cho DH. Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta Indica A. Juss grown in foothills of Nepal. African J Biotechnol. 2009;8(13):3084-3091.
Yerima M, Jodi S, Oyinbo K, et al. Effect of neem extracts (Azadirachta indica) on bacteria isolated from adult mouth. Niger J Basic Appl Sci. 2012;20(1):64-67.
Okemo P, Mwatha W, Chhabra S, Fabry W. The kill kinetics of azadirachta indica a. juss.(meliaceae) extracts on staphylococcus aureus, escherichia coli, pseudomonas aeruginosa and candida albicans. AJST. 2001;2(2):113-118.
Sinha DJ, Nandha KD, Jaiswal N, Vasudeva A, Tyagi SP, Singh UP. Antibacterial effect of Azadirachta indica (Neem) or Curcuma longa (Turmeric) against Enterococcus faecalis compared with that of 5% sodium hypochlorite or 2% chlorhexidine in vitro. Bull Tokyo Dent Coll. 2017;58(2):103-109. https://doi.org/10.2209/tdcpublication.2015-0029
Khan S, Imran M, Imran M, Pindari N. Antimicrobial activity of various ethanolic plant extracts against pathogenic multi drug resistant Candida spp. Bioinformation. 2017;13(3):67-72. https://doi.org/10.6026/97320630013067
Copyright (c) 2022 Sumaira Mazhar, Waqar Saleem, Beenish Sarfraz

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










