Gelatin extracted from rahu (Labeo rohita) and silver carp (Hypophthalmichthysmolitrix) scales and their beads efficacy as a carrier of inoculum and secondary metabolites
Abstract
 Abstract Views: 186
Abstract Views: 186
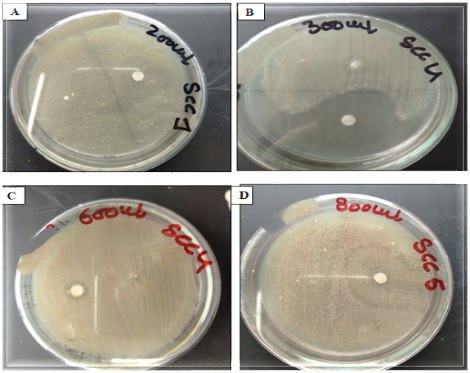
Nowadays biological waste is increasing due to over population. At the same time, researchers are trying to use biological wastes in effective ways and trying to convert these wastes into useful products. In this study the scales of rohu (Labeo rohita) and silver carp (Hypophthalmichthys molitrix) were used for the extraction of gelatin. Chemical extraction method was mediated in the presence and absence of microbes to obtain gelatin from varying amounts of scales. Microbial isolates SCC5 from Silver carp and SEY from Labeo rohita were used for microbial extraction. Beads were prepared from extracted gelatin using three different types of oils and later used as a carrier of inoculum. Results showed that both isolates were positive for gelatin liquefaction liquifection and did not result in solidification even at low temperature when kept in the refrigerator. The amount of extracted gelatin from fish scales was less than 50 %. A pronounced increase was recorded when more than 30 gram of fish scales were used for extraction. It was noticed that beads prepared with natural gelatin were remained stable after washing and not degraded as compared to commercial gelatin. FTIR analysis of extracted gelatin showed the presence of peaks at 700, 1600 and 3400 nm range that were in line with peak trends of commercial gelatin. Beads were coated with antibiotic by soaking in the antibiotic dilution. From the zones formation of beads supplemented with the antibiotics it was observed that zone diameter increased as amount of antibiotic increased it means the beads have the capacity to carry the antibiotics. Moreover, t and beads of extracted gelatin were injected with the different inoculum of bacterial isolatesgelatin beads can also be used as carrier of fertilizers in agriculture, which is a recent advancement in the field of agriculture.
Downloads
References
Jarwar AA. A status overview of fisheries and aquaculture development in Pakistan with context to other Asian countries. Aquaculture Asia Magazine, 2008.
Laghari MY. Aquaculture in Pakistan: Challenges and opportunities. Int J Fish Aquatic Stud. 2018;6(2):56-59.
Hussain J, Rabbani I, Aslam S, Ahmad H. An overview of poultry industry in Pakistan. World's Poult Sci J. 2015;71(4):689-700.
Ranjan A. The China-Pakistan economic corridor: Options before India. Institute of Chinese Studies. 2015;10(1):1-25.
Mariod AA, Fadul H. Gelatin, source, extraction and industrial applications. Acta Sci Pol Technol Aliment. 2013;12(2):135-147.
Hamidi-Asl E, Dardenne F, Blust R. De Wael K. An improved electrochemical aptasensor for chloramphenicol detection based on aptamer incorporated gelatine. Sensors. 2015;15(4):7605-7618.
Shyni K, Hema G, Ninan G, Mathew S, Joshy C, Lakshmanan P. Isolation and characterization of gelatin from the skins of skipjack tuna (Katsuwonus pelamis), dog shark (Scoliodon sorrakowah), and rohu (Labeo rohita). Food Hydrocoll. 2014;39:68-76. https://doi.org/10.1016/j.foodhyd.2013.12.008
Okuyama K. Miyama K. Mizuno K. Bächinger HP. Crystal structure of (Gly‐Pro‐Hyp) 9: Implications for the collagen molecular model. Biopolymers. 2012;97(8):607-616. https://doi.org/10.1002/bip.22048
Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78: 929-958. https://doi.org/10.1146/annurev.biochem.77.032207.120833
Haug IJ, Draget KI, Smidsrød O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004;18(2):203-213. https://doi.org/10.1016/S0268-005X(03)00065-1
Rbii K. Formation of high molecular weight aggregates in gelatin and behavior in aqueous solution [dissertation]. Institut National Polytechnique de Toulouse; 2010.
Jones RT. Gelatin: Manufacture and physico-chemical properties. London, UK, Pharmaceutical Press; 2004.
Duconseille A, Astruc T, Quintana N, Meersman F, Sante-Lhoutellier V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015;43:360-376. https://doi.org/10.1016/j.foodhyd.2014.06.006
Basu S, Bose C, Ojha N, et al. Evolution of bacterial and fungal growth media. Bioinformation. 2015;11(4):182-184.
Hassan EM, Fatmi AA, Chidambaram N. Enteric composition for the manufacture of soft. Google Patents. https://patents.google.com/patent/EP1545475A1/en, 2014.
Pogue BW, Patterson MS. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J Biomed Opt. 2006;11(4):e041102. https://doi.org/10.1117/1.2335429
James C. The book of alternative photographic processes. Boston, USA, Cengage Learning; 2015.
Ramos M, Valdes A, Beltran A, Garrigós M. Gelatin-based films and coatings for food packaging applications. Coatings. 2016;6(4):e41. https://doi.org/10.3390/coatings6040041
Ul Rehman W, Majeed A, Mehra R, et al. Gelatin: A comprehensive report covering its indispensable aspects. Nova Science Publishers, Inc.;2016.
Nik Aisyah N, Nurul H, Azhar M, Fazilah A. Poultry as an alternative source of gelatin. Health Environ J. 2014;5(1):37-49.
Eriksson A, Burcharth J, Rosenberg J. Animal derived products may conflict with religious patients’ beliefs. BMC Med Ethics. 2013;14(1):e48. https://doi.org/10.1186/1472-6939-14-48
Zakaria S, Bakar NHA. Extraction and characterization of gelatin from Black tilapia (Oreochromis niloticus) scales and bones. Paper presented at the Kota Kinabalu (MY): Paper presented at: International Conference On Advances in Science, Engineering, Technology And Natural Resources; August 27-28, 2015, Kota Kinabalu, Malaysia. https://iicbe.org/upload/3199C0815040.pdf
Lin CC, Chiou TK, Sung WC. Characteristics of gelatin from giant grouper (Epinephelus Lanceolatus) skin. Int J Food Prop. 2015;18(11):2339-2348. https://doi.org/10.1080/10942912.2014.980947
Zhang QT, Tu ZC, Xiao H, et al. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food Biopro Processing. 2014;92(1):30-37. https://doi.org/10.1016/j.fbp.2013.07.006
Wangtueai S, Noomhorm A. Processing optimization and characterization of gelatin from lizardfish (Saurida spp.) scales. LWT-Food Sci Technol. 2009;42(4):825-834. https://doi.org/10.1016/j.lwt.2008.11.014
López‐Velázquez JC, Rodríguez‐Rodríguez R, Espinosa‐Andrews H, et al. Gelatin–chitosan–PVA hydrogels and their application in agriculture. J Chem Technol Biotechnol. 2019;94(11):3495-3504. https://doi.org/10.1002/jctb.5961
Das M, Suguna P, Prasad K, Vijaylakshmi J, Renuka M. Extraction and characterization of gelatin: a functional biopolymer. Int J Pharm Sci. 2017;9(1):239-242.
dela Cruz TEE, Torres JMO. Gelatin hydrolysis test protocol. American Society for Microbiology; 2012. https://link.springer.com/content/pdf/10.1007/s13213-019-01533-z.pdf
Iwamoto S, Nakagawa K, Sugiura S, Nakajima M. Preparation of gelatin microbeads with a narrow size distribution using microchannel emulsification. AAPS Pharm Sci Tech. 2002;3(3):72-76. https://doi.org/10.1208/pt030325
Nazir K, Yongtong M, Kalhoro MA, Memon KH, Mohsin M, Kartika S. A preliminary study on fisheries economy of Pakistan: Plan of actions for fisheries management in Pakistan. Can J Basic Appl Sci. 2015;3(01):7-17.
DeRuiter, J. (2005). Amides and related functional groups. Principles of Drug Action, 1, 1-16.
Herpandi H, Huda N, Adzitey F. Fish bone and scale as a potential source of halal gelatin. J Fish Aquatic Sci. 2011;6(4):379-389.
Baziwane D, He Q. Gelatin: The paramount food additive. Food Rev Int. 2003;19(4):423-435. https://doi.org/10.1081/FRI-120025483
Bichukale A, Koli J, Sonavne A, Vishwasrao V, Pujari K, Shingare P. Functional properties of gelatin extracted from poultry skin and bone waste. Int J Pure App Biosci. 2018;6(4):87-101. http://dx.doi.org/10.18782/2320-7051.6768
Sahoo N, Sahoo RK, Biswas N, Guha A, Kuotsu K. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int J Biol Macromol. 2015;81(1):317-331. https://doi.org/10.1016/j.ijbiomac.2015.08.006
Su K, Wang C. Recent advances in the use of gelatin in biomedical research. Biotechnol Letters. 2015;37(11):2139-2145. https://doi.org/10.1007/s10529-015-1907-0
Foox M, Zilberman M. Drug delivery from gelatin-based systems. Expert Opin Drug Deliv. 2015;12(9):1547-1563. https://doi.org/10.1517/17425247.2015.1037272
Bashan Y. Alginate beads as synthetic inoculant carriers for slow release of bacteria that affect plant growth. Appl Environ Microbiol. 1986;51(5):1089-1098. https://doi.org/10.1128/aem.51.5.1089-1098.1986
Schirawski J, Perlin MH. Plant–microbe interaction 2017—the good, the bad and the diverse. Intl J Molecul Sci. 2018;19(5):e1374. https://doi.org/10.3390/ijms19051374
Copyright (c) 2022 Dr. Roheela Yasmeen, Dr. Aisha Waheed Qurashi, Samreen Arif, Hina Qaiser

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










