Antibiogram Analysis of Salmonella paratyphi A Isolated from Gall Bladder Patients in District Peshawar, Pakistan
Abstract
 Abstract Views: 108
Abstract Views: 108
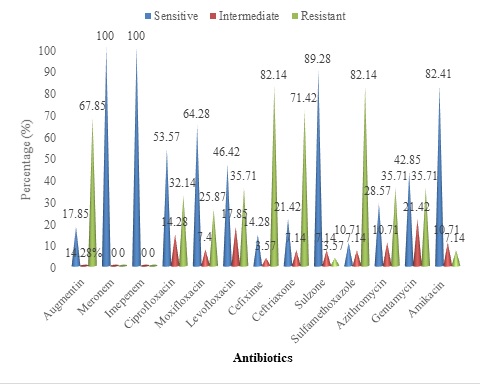
Salmonella paratyphi A harbors gall bladder in the human body. It serves as a site of persistence for Salmonella paratyphi A. It is an enteric pathogen which has become resistant to many drugs. Therefore, the current study was designed for the identification and antibiogram analysis of S. paratyphi A, isolated from the gall bladder patients undergone cholecystectomy. It included 250 samples of bile, stone, and tissue of patients. The samples were cultured on blood, macConkey, and Salmonella Shigella media. Further identification was carried out by morphological oxidase test and Analytical Profile Index (API) strips, followed by antibiogram analysis of the isolates. In the current study, twenty-eight (11.2%) paratyphi A were isolated including 10 (10%) from male patients and 18 (12%) from female patients. Furthermore, 96 samples were found to be positive for miscellaneous growth including 53 with S. typhi (21.2%), 13 with Escherichia coli (5.2%), 09 with Klebsiella (3.6%), 07 with Providencia (2.8%), 05 with Pseudomonas (2%), 03 with Proteus (1.2%), and 06 with Staphylococcus aureus (2.4%). The distribution and susceptibility pattern of S. paratyphi A isolates was checked in different types of clinical specimens including bile, stones, tissue, bile/stones, bile/tissue, stones/tissue, and bile/stone/tissue. S. paratyphi A was distributed as follows: bile (11), stones (5), tissue (3), bile/ stones (4), stones/ tissue (1), bile/tissue (1), and bile/stones/tissue (3). The results of the antibiogram analysis found that the isolates of Paratyphi A were resistant to sulfamethoxazole 23 (82.14%), cefixime 23 (82.14%), ceftriaxone (rocephin) 20 (71.42%), augmentin 19 (67.85%), and azithromycin 18 (64.28%). The increased susceptibility of these isolates was towards meronem 28 (100%), imipenem 28 (100%), cefoperazon + sulbactam (sulzone) 25 (89.28%), and amikacin 23 (82.14%). The current study signifies the use of the Antibiogram Analysis of Salmonella paratyphi A most susceptible and effective antibiotic options for gall bladder diseases complicated by S. paratyphi A, which showed resistance to ceftriaxone (rocephin), cefixime, sulfamethoxazole, azithromycin, and augmentin, while sensitivity to meropenem, imipenem, cefoperazone + sulbactam (sulzone), and amikacin. It makes the latter a better choice for treatment against the gall stone disease complicated with S. paratyphi A infection.
Downloads
References
D'Aoust J, Maurer J, Baile JS, Doyle M, Beuchat L. Salmonella species. J Food Microbiol Fundament Front. 2001;3:187-237.
Prieto AI, Ramos MF, Casadesús, J. Repair of DNA damage induced by bile salts in Salmonella enterica. Genetics. 2006;174(2):575-584. https://doi.org/10.1534/genetics.106.060889
Schoenwolf GC, Bleyl SB, Brauer PR, Francis-West PH. Elevisier Larsen's human embryology. Development of the gastrointestinal tract. 4th ed. Churchill Livingstone; 2009.
Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. J Microbiol Rev. 2005;29(4): 625-651. https://doi.org/10.1016/j.femsre.2004.09.003
Escobedo GG, Marshall JM, Gunn JS. Chronic and acute infection of the gall bladder by Salmonella Typhi: Understanding the carrier state. J Nature Rev Microbiol. 2011;9:9-14. https://doi.org/10.1038/nrmicro2490
Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. New England J Med. 2002;347:1770–1782.
Gal-Mor O, Suez J, Elhadad D, et al. Molecular and cellular characterization of a Salmonella enterica serovar paratyphi A outbreak strain and the human immune response to infection. J Clinic Vacc Immunol. 2012;19(2):146-156. https://doi.org/10.1128/CVI.05468-11
Crump JA, Luby SP Mintz ED. The global burden of typhoid fever. J Bull World Health Org. 2004;82(5):346–353.
Gomes PRL, Fernando SSN, Weerasekara DD, et al. Aerobic bacteria associated with symptomatic gallstone disease and their antimicrobial susceptibility. Gall Med J. 2006;11(1):9-13.
Raphael R, Strayer DS, Rubin E, McDonald JM. Rubin's pathology. 5th ed. Wolters Kluwer Health; 2007.
Bhattacharya SS, Das U, Choudhury BK. Occurrence and antibiogram of Salmonella Typhi and S. paratyphi A isolated from Rourkela, Orissa. Ind J Med Microbiol. 2011;133(4):431-433.
Dongol S, Thompson CN, Clare S, et al. The microbiological and clinical characteristics of invasive Salmonella in gallbladders from cholecystectomy patients in Kathmandu, Nepal. J Pub Lib Sci. 2012.;7(10):47342.
Chen S, Cui S, McDermott PM. Contribution of target gene mutations and efflux to decreased susceptibility of Salmonella enterica serovar Typhimurium to fluoroquinolones and other antimicrobials. J Antimicro Agents Chemoth. 2007;51:535–542. https://doi.org/10.1128/AAC.00600-06
Stock I, Wiedemann B. Natural antibiotic susceptibility of Salmonella enteric strains. Int J Antimicrob Agents. 2000;16(3):211-217. https://doi.org/10.1016/S0924-8579(00)00204-1
Orman B, Piñeiro SA, Arduino S, et al. Evolution of multi resistance in no typhoid Salmonella serovars from 1984 to 1998 in Argentina. J Antimicrob Agents Chemother. 2002;46(2):3963-3970. https://doi.org/10.1128/AAC.46.12.3963-3970.2002
Miko A, Pries K, Schroeter A, Helmuth R. Molecular and cellular characterization of a Salmonella enterica serovar paratyphi A outbreak strain and the human immune response to infection. J Antimicrob Chemother. 2005;56(6):1025-1033. https://doi.org/10.1093/jac/dki365
Hakanen A, Kotilainen P, Huovinen P, Helenius H, Siitonens A. Reduced fluoroquinolone susceptibility in Salmonella enterica serotypes in travelers returning from South East Asia. J Emerg Infec Dis. 2001;7:996-1003. https://doi.org/10.3201/eid0706.010613
Menezes GA, Harish BN, Khan MA, Goessens WH, Hays JP. Antimicrobial resistance trends in blood culture positive Salmonella Typhi isolates from Pondicherry, India, 2005–2009. J Clinic Microbiol Infec. 2011;18(3):239–245. https://doi.org/10.1111/j.1469-0691.2011.03546.x
Pokharel BM, Koirala J, Dahal RK, Mishra SK, Khadga PK, Tuladhar NR. Multidrug-resistant and extended-spectrum beta-lactamase (ESBL)-producing Salmonella enterica (serotypes typhi and paratyphi A) from blood isolates in Nepal: Surveillance of resistance and a search for newer alternatives. Int J Infec Dis. 2006;10(6):434-438. https://doi.org/10.1016/j.ijid.2006.07.001
Vlieghe E, Phe T, Dee SB, et al. Increase in Salmonella enterica serovar paratyphi A infections in Phnom Penh, Cambodia, January 2011 to August 2013. Euro Surveill. 2013;18(39):e20592.
Ploy MC, Chainier C, Tran TNH, Poilane I, Cruaud P. Integron-associated antibiotic resistance in Salmonella enterica serovar Typhi from Asia. J Antimicrob Agents Chemother. 2003;47:1427-1429. https://doi.org/10.1128/AAC.47.4.1427-1429.2003
Piddock LJV, Garvey MI, Rahman MM, Gibbons S. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in Gram negative bacteria. J Antimicrob Chemother. 2010;65(6):1215–1223. https://doi.org/10.1093/jac/dkq079
Rupali P, Abraham OC, Jesudason MV. Treatment failure in typhoid fever with ciprofloxacin susceptible Salmonella enterica serotype Typhi. J Diagnostic Microbiol Infec Dis. 2004;49(1):1-3. https://doi.org/10.1016/j.diagmicrobio.2003.12.002
Abid AJ, Kadhim SJ. Bacteriological and immunological study of cholecystectomy patients. J Babylon Univ/Pure Appl Sci. 2014;22(2):809-826.
Prouty AM, Schwesinger WH, Gunn JS. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. J Infect Immunol. 2002;70(5):2640-2649. https://doi.org/10.1128/IAI.70.5.2640-2649.2002
Capoor MR, Nair D, Khanna G, Krishna SV, Chintamani MS, Aggarwal P. Microflora of bile aspirates in patients with acute cholecystitis with or without cholelithiasis: a tropical experience. Braz J Infec Dis. 2008;12:222-225. https://doi.org/10.1590/S1413-86702008000300012
Eslami G, Nowruzi J, Fllah F, Goudarzi H, Hakemivala M, Jahangiri S. Detection of bacteria responsible for gallbladder inflammation and gallstones. Iran J Clinic Infec Dis. 2007;2(3):139-141.
Manan F, Atif KM, Faraz A, Khan M. Frequency of common bacteria and their anti¬biotic sensitivity in patients with symptomatic cholelithiasis. J Postgrad Med Inst. 2014;28(2):177-83.
Khatari NS. Gall bladder carriage of Salmonella paratyphi A may be an important factor in the increasing incidence of this infection in south Asia. Int J Med. 2009;150(5):567-568. https://doi.org/10.7326/0003-4819-150-8-200904210-00017
Latif M, Gilani M, Usman J, Munir T, Mushtaq M, Babar N. Lactose fermenting Salmonella paratyphi A: A case report. J Microbiol Infect Dis. 2014;4(1):30-32. https://doi.org/10.5799/ahinjs.02.2014.01.0120
Kaya M, Bestas R, Bacalan F, Bacaksız F, Arslan EG, Kaplan MA. Microbial profile and antibiotic sensitivity pattern in bile cultures from endoscopic retrograde cholangiography patients. World J Gastroenterol. 2012;18(27):3585-3589.
Dutta S, Das S, Mitra U, et al. Antimicrobial resistance, virulence profiles and molecular subtypes of Salmonella enterica serovars Typhi and paratyphi A blood isolates from Kolkata, India during 2009-2013. PLoS One. 2014;9(8):e101347. https://doi.org/10.1371/journal.pone.0101347
Ahmad F, Islahi S, Musa HO, Singh YI. Cholelithiasis: A clinical and microbiological analysis. Int J Sci Study. 2014;2(4):41-45.
Raji MA, Aluwong M. Multi-drug resistance Salmonella species in human and use of medicinal plants in Nigeria. Glob Res J Microbiol. 2011;1(1):1-4.
Butler T, Sridhar CB, Daga MK, Pathak K, Pandit RB, Khakhria R. Treatment of typhoid fever with Azithromycin versus chloramphenicol in a randomized multicentre trial in India. J Antimicrob Chemother. 1999;44:243-250. https://doi.org/10.1093/jac/44.2.243
Capoor MR, Nair D, Hasan AS, Agarwal P, Gupta B. Typhoid fever: Narrowing therapeutic options in India. Southeast Asian J Tropic Med Pub Health. 2007;37(6):1170-1174.
Rizvi Q. Effectiveness of antityphoidal drugs currently used in Pakistan. Pak J Surg. 2007;23(1):57-64.
Toh HS, Chuang YC, Huang CC, et al. Antimicrobial susceptibility profiles of Gram-negative bacilli isolated from patients with hepatobiliary infections in Taiwan: Results from the Study for monitoring antimicrobial resistance trends (SMART), 2006–2010. Int J Antimicrob Agents. 2012;40(1):S18-S23. https://doi.org/10.1016/S0924-8579(12)70005-5
Stass H, Kubitza D, Halabi A. Pharmacokinetic of moxifloxacin, a novel 8- methoxy- quinolone in patients with renal dysfunction. Br J Clin Pharmacol. 2002;53(3):233-237. https://doi.org/10.1046/j.0306-5251.2001.01557.x
Garg A, Anupurba S, Jaya G, Goyal RK, Sen MR. Bacteriological profile and antimicrobial resistance of blood culture isolates from a university hospital. J Ind Acad Clinl Med. 2007;8(2):139-143.
Copyright (c) 2022 Muhammad Nazir Uddin, Alveena Mukhtiar, MUDDASIR KHAN, Ghadir Ali, Wajid Khan, Taj Uddin

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










