Popular Influenza Antiviral Drugs: Mechanisms, Efficacy, and Resistance
Abstract
 Abstract Views: 0
Abstract Views: 0
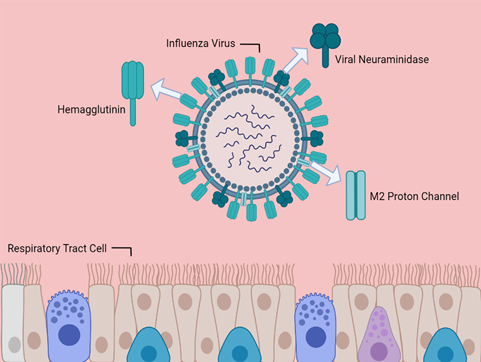
Influenza viruses cause acute respiratory infections responsible for significant mortality and morbidity around the world. Various factors, such as antigenic drift, allow influenza strains to avoid being fully suppressed by seasonal vaccines. This has led to the increased scrutiny of antivirals as treatment and prophylaxis options for seasonal outbreaks and potential pandemics. Unfortunately, many influenza antivirals suffer from a lack of adequate clinical trials, as well as a lack of toxicity data. This is especially true of umifenovir (arbidol), a drug popularly used for the prevention and treatment of influenza strains in China and Russia. Neuraminidase inhibitors, though widely prescribed, display a potential for future resistance. Adamantanes, while proven to be effective in treating influenza A, are already encountering rapid and widespread cross-resistance and are effectively obsolete. Baloxavir marboxil, a newer antiviral, shows promise in treating acute uncomplicated influenza and may avoid the development of resistance when co-administered with other antiviral drugs. Indeed, the low genetic barrier to resistance associated with influenza antivirals could potentially be overcome by co-administration with other antivirals. This review explores the most widely prescribed antivirals for influenza treatment, their mechanisms of action, and the data currently available about their susceptibility to resistance and efficacy.
Downloads
References
Fukao, K., Noshi, T., Yamamoto, A., Kitano, M., Ando, Y., Noda, T., . . . Naito, A. (2018). Combination Treatment with the Cap-Dependent Endonuclease Inhibitor Baloxavir Marboxil and a Neuraminidase Inhibitor in a Mouse Model of Influenza A Virus Infection. Journal of Antimicrobial Chemotherapy, 74(3), 654-662. doi:10.1093/jac/dky462
Haviernik, J., Štefánik, M., Fojtíková, M., Kali, S., Tordo, N., Rudolf, I., . . . Ruzek, D. (2018). Arbidol (Umifenovir): A Broad-Spectrum Antiviral Drug That Inhibits Medically Important Arthropod-Borne Flaviviruses. Viruses, 10(4), 184. doi:10.3390/v10040184
Images of Influenza Viruses CDC. (2014, June 4). Retrieved form https://www.cdc.gov/flu/resource-center/freeresources/graphics/images.htm
Influenza (Flu) CDC. (2022, May 12). Retrieved from https://www.cdc.gov/flu/index.htm
Ison, M. G., Hayden F. G. (2017). Antiviral Agents against Respiratory Viruses. In C. Agustí and A Martí (Eds.), Pulmonary Infection in the Immunocompromised Patient (pp. 401-428). doi:10.1002/9780470714171.ch15
Jefferson, T., Jones, M. A., Doshi, P., Mar, C. B., Hama, R., Thompson, M. J., . . . Heneghan, C. J. (2014). Neuraminidase Inhibitors for Preventing and Treating Influenza in Adults and Children. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.cd008965.pub4
Koel, B. F., Mögling, R., Chutinimitkul, S., Fraaij, P. L., Burke, D. F., Vliet, S. V., . . . Graaf, M. D. (2015). Identification of Amino Acid Substitutions Supporting Antigenic Change of Influenza A(H1N1)pdm09 Viruses. Journal of Virology, 89(7), 3763-3775. doi:10.1128/jvi.02962-14
Leneva, I. A., Falynscova, I. N., Leonova, E. I., Fedyakina, I. T., Makhmudova, N. R., Osipova, E. A., . . . Zverev, V. V. (2014). Umifenovir (Arbidol) Efficacy in Experimental Mixed Viral and Bacterial Pneumonia of Mice [Abstract]. Antibiotics and Chemotherepy, 59(9- 10), 17-24.
Mondal, D. (2016). Rimantadine☆. Reference Module in Biomedical Sciences. doi:10.1016/b978-0-12-801238-3.99410-8
Moscona, A. (2005). Neuraminidase Inhibitors for Influenza. New England Journal of Medicine, 353(13), 1363-1373. doi:10.1056/nejmra050740
Paget, J., Spreeuwenberg, P., Charu, V., Taylor, R. J., Iuliano, A. D., Bresee, J., Simonsen, L., Viboud, C., & Global Seasonal Influenza-associated Mortality Collaborator Network and GLaMOR Collaborating Teams* (2019). Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. Journal of global health, 9(2), 020421. https://doi.org/10.7189/jogh.09.020421
Portsmouth, S., Kawaguchi, K., Arai, M., Tsuchiya, K., & Uehara, T. (2017). Cap-dependent Endonuclease Inhibitor S-033188 for the Treatment of Influenza: Results from a Phase 3, Randomized, Double-Blind, Placebo- and Active-Controlled Study in Otherwise Healthy Adolescents and Adults with Seasonal Influenza. Open Forum Infectious Diseases, 4(Suppl_1). doi:10.1093/ofid/ofx180.001
Retamal, M., Abed, Y., Rhéaume, C., Baz, M., & Boivin, G. (2017). In vitro and in vivo Evidence of a Potential A(H1N1)pdm09 Antigenic Drift Mediated by Escape Mutations in the Haemagglutinin Sa Antigenic Site. Journal of General Virology, 98(6), 1224-1231. doi:10.1099/jgv.0.000800
Shi, L., Xiong, H., He, J., Deng, H., Li, Q., Zhong, Q., . . . Yang, Z. (2007). Antiviral Activity of Arbidol against Influenza A Virus, Respiratory Syncytial Virus, Rhinovirus, Coxsackie Virus and Adenovirus in vitro and in vivo. Archives of Virology, 152(8), 1447-1455. doi:10.1007/s00705-007-0974-5
Yang, T. (2019). Baloxavir Marboxil: The First Cap-Dependent Endonuclease Inhibitor for the Treatment of Influenza. Annals of Pharmacotherapy, 106002801982656. doi:10.1177/1060028019826565
Copyright (c) 2023 Sidney Ley

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










