Morpho-Molecular Study and Biochemical Management of Fusarium Solani in Bell Pepper
Abstract
 Abstract Views: 0
Abstract Views: 0
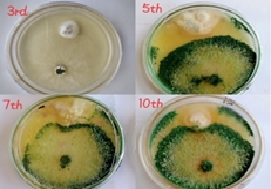
Bell pepper (Capsicum annum L.) is one of the most important vegetable crops in Pakistan. This crop is prone to many fungal diseases, such as Phytophthora blight, Fusarium wilt, Verticillium wilt, Powdery mildew, and Anthracnose. Among all of them, Fusarium wilt is the most devastating disease of bell pepper that is responsible for heavy yield reduction. This study is aimed to identify and manage Fusarium solani in bell pepper, in vitro as well as in pots experiment. For this purpose, sampling, isolation, purification, and morpho-molecular identification were performed. Molecular characterization of F. solani was achieved by using ITS primers. The results showed that the nucleotide sequence of these isolates showed 100% homology to Fusarium solani. In vitro management was done by the application of Trichoderma harzianum and synthetic fungicides (Thiophanate methyl, Mancozeb+Metalaxyl, Fosetyl aluminum, Difenoconazole, and Sulphur). Five concentrations (100 ppm, 200 ppm, 400 ppm, 800 ppm, and 1000 ppm) were employed using dual culture and food poisoning method, respectively. LSD was used in combination with four replications. Data was taken after 3, 5, 7, and 10 days. The results showed that T. harzianum inhibited F. solani growth up to 87.98% after 10 days. As compared to the biological treatment, Thiophanate methyl showed maximum inhibition (100%) after 3 days at 100 ppm. Later on, its efficacy at 100 ppm was reduced after 5, 7, and 10 days. The inhibition after 10 days was calculated to be 72.89%. At 200, 400, 800, and 1000 ppm, the percentage of inhibition was up to 100%. Furthermore, Mancozeb + Metalaxyl showed maximum inhibition (100%) after 3 days at 1000 ppm. Similarly, Fosetyl aluminum showed maximum inhibition (100%) after 3 days at 1000 ppm. Whereas, Difenoconazole showed maximum inhibition (100%) after 3 days at 800 ppm. Later on, the efficacy at 800 ppm was reduced after 7 and 10 days. The inhibition after 10 days was calculated to be 87.72%. At 1000 ppm, the percentage of inhibition was up to 100%. Finally, sulphur showed maximum inhibition 82.7% after 3 days at 1000 ppm. Later on, its efficacy at 1000 ppm was reduced after 5, 7, and 10 days. The inhibition after 10 days was calculated to be 62.62%.
Downloads
References
Sreedhara DS, Kerutagi MG, Basavaraja H, Kunnal LB, Dodamani MT. Economics of Capsicum production under protected conditions in Northern Karnataka. Karnataka J Agricul Sci. 2013;26(2):217–219.
Hussain F, Abid M. Pest and diseases of Chilli crop in Pakistan: a review. Int J Biol Biotech. 2011;8(2):325–332.
Durucasu I, Tokuşoğlu O. Effects of grilling on luteolin (3', 4', 5, 7-tetrahydroxyflavone) content in sweet green bell pepper (Capsicum annuum). Pak J Biol Sci. 2007;10(19):3410–3414. https://doi.org/10.3923/pjbs. 2007.3410.3414
Hallmann E, Rembiałkowska E. Characterisation of antioxidant compounds in sweet bell pepper (Capsicum annuum L.) under organic and conventional growing systems. J Sci Food Agric. 2012;92(12):2409–2415. https://doi.org/10.1002/jsfa .5624
Kothari SL, Joshi A, Kachhwaha S, Ochoa-Alejo N. Chilli peppers—a review on tissue culture and transgenesis. Biotechnol Adv. 2010;28(1):35–48. https://doi.org/10. 1016/j.biotechadv.2009.08.005
Katoch A, Kapoor P. Fungal diseases of Capsicum and their management. Popular Kheti. 2014;2(2):100–103.
Verma C, Gupta BK, Singh A, Kujur A, Sahu R, Prajapati P. Biological control agents in the management of bell pepper nursery diseases: a review. J Pharmacogn Phytochem. 2020;9:462–468.
Shali S. Studies on Chilli Wilt in Jammu [dissertation]. Sher-e-Kashmir University of Agriculture Science and Technology; 2000.
Najar AG. Cause and Management of Chilli Wilt in Kashmir [Doctoral dissertation]. Sher-e-Kashmir University of Agricultural Science and Technology; 2001.
Amorim L, Rezende JAM, Filho A, Bergamin, Camargo LEA. Manual 489 of Phytopathology: Diseases of Cultivated Plants. 5th ed. Ceres Agronomy; 2016.
Mamta J, Rashmi S, Anil KS, Anil P. Screening of resistant varieties and antagonistic fusarium Oxysporum for biocontrol of fusarium wilt of Chilli. J Plant Pathol Microbiol. 2012;3(5):e1000134. https://doi.org/ 10.4172/2157-7471.1000134
Sela-Buurlage M, Budai-Hadrian O, Pan Q, et al. Genome-wide dissection of Fusarium resistance in tomato reveals multiple complex loci. Mol Genet Genom. 2001;265(6):1104–1111. https://doi.org/10.1007/s004380 100509
Brewer MT, Larkin RP. Efficacy of several potential biocontrol organisms against Rhizoctonia solani on potato. Crop Prot. 2005;24(11):939–950. https://doi.org/10.1016/j.cropro.2005.01.012
Akram W, Mahboob A, Javed AA. Bacillus thuringiensis strain 199 can induce systemic resistance in tomato against Fusarium wilt. Eur J Microbiol Immunol. 2013;3(4):275–280. https:// doi.org/10.1556/eujmi.3.2013.4.7
Zaher EA, Abada KA, Zyton MA. Effect of combination between bioagents and solarization on management of crown-and stem-rot of Egyptian clover. J Plant Sci. 2013;1(3):43–50.
Abada KA, Eid KE. A protocol suggested for management of cantaloupe downy mildew. Am J Life Sci. 2014;2:1–10. https://doi.org/10. 11648/j.ajls.s.2014020602.11
Ozbay N, Newman SE. Effect of Trichoderma harzianum strains to colonize tomato roots and improve transplant growth. Pak J Biol Sci. 2004;7(2):253–257.
White TJ, Bruns T, Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds., PCR Protocols: A Guide to Methods and Applications. Academic Press; 1990:315–322.
Baba ZA, Padder SA, Bhat MA, et al. Incidence of Fusarium wilt of chilli (Capsicum annum L.) in Kashmir valley and its management by Trichoderma spp. Mycopath. 2014;12:1–8.
Ortoneda M, Guarro J, Madrid MP, et al. Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infec Immun. 2004;72(3):1760–1766. https://doi. org/10.1128/iai.72.3.1760-1766.2004
Mezzomo R, Rolim JM, Poletto T, et al. Morphological and molecular characterization of Fusarium spp. pathogenic to Ilex Paraguariensis. Cerne. 2018;24:209–218.
Ferniah RS, Daryono BS, Kasiamdari RS, Priyatmojo A. Characterization and pathogenicity of Fusarium oxysporum as the Causal Agent of Fusarium wilt in chili (Capsicum annuum L.). Microbiol Indone. 2014;8(3):121–126. https://doi.org/10. 5454/mi.8.3.5
Jeon CS, Kim GH, Son KI, et al. Root rot of balloon flower (Platycodon grandiflorum) caused by Fusarium solani and Fusarium oxysporum. Plant Pathol. J. 2013;29(4):440–445. https:// doi.org/10.5423/PPJ.NT.07.2013.0073
Singha IM, Kakoty Y, Unni BG, Das J, Kalita MC. Identification and characterization of Fusarium sp. using ITS and RAPD causing Fusarium wilt of tomato isolated from Assam, North East India. J Genet Eng Biotechnol. 2016;14(1):99–105. https://doi.org/ 10.1016/j.jgeb.2016.07.001
Khan MA, Khan SA, Waheed U, et al. Morphological and genetic characterization of Fusarium oxysporum and its management using weed extracts in cotton. J King Saud Uni Sci. 2021;33(2):e101299. https://doi.org/10.1016/j.jksus.2020.101299
Khaledi N, Taheri P. Biocontrol mechanisms of Trichoderma harzianum against soybean charcoal rot caused by Macrophomina Phaseolina. J Plant Prot Res. 2016;56(1):21–31.
Aswini A, Sharmila T, Raaga K, Sri Deepthi R, Krishna MS. In vitro antifungal activity of Trichoderma strains on pathogenic fungi inciting hot pepper (Capsicum annuum L.). J Chem Pharm Res. 2016;8(4):425–430.
Miguel-Ferrer L, Romero-Arenas O, Andrade-Hoyos P, Sánchez-Morales P, Rivera-Tapia JA, Fernández-Pavía SP. Antifungal activity of Trichoderma harzianum and T. koningiopsis against Fusarium solani in seed germination and vigor of Miahuateco chili seedlings. Mex J Phytopathol. 2021;39(2):228–247. https://doi.org/ 10.18781/r.mex.fit.2101-5
Anjum NA, Shahid AA, Iftikhar SE, et al. Evaluations of Trichoderma isolates for biological control of Fusarium wilt of chili. Plant Cell Biotechnol Mol Biol. 2020;21(59-60):42–57.
Akrami M, Yousefi Z. Biological control of Fusarium wilt of tomato (Solanum lycopersicum) by Trichoderma spp. as antagonist fungi. Biol Forum. 2015;7(1): 887–892.
Ghanbarzadeh B, Safaie N, Goltapeh EM. Antagonistic activity and hyphal interactions of Trichoderma spp. against Fusarium proliferatum and F. oxysporum in vitro. Arch Phytopathol. 2014;47(16):1979–1987. https://doi. org/10.1080/03235408.2013.864506
Cherkupally R, Amballa H, Reddy BN. In vitro antagonistic activity of Trichoderma species against Fusarium oxysporum f. sp. melongenae. Int J Appl Agric Res. 2017;12(1):87–95.
Dar GH, Mir GH, Rashid H, Dar WA, Majeed M. Evaluation of microbial antagonists for the management of wilt/root rot and damping-off diseases in chilli (Capsicum annuum). Vegetos. 2015;28(4):102–110.
Sahi IY. In vitro biological control of Fusarium oxysporum-causing wilt in Capsicum annuum. Mycopath. 2007;5(2):85–88
Ragab MM, Ashour AM, Abdel-Kader MM, El-Mohamady R, Abdel-Aziz A. In vitro evaluation of some fungicides alternatives against Fusarium oxysporum the causal of wilt disease of pepper (Capsicum annum L.). Int J Agric Forest. 2012;2(2):70–77. https://doi.org/10.5923/j.ijaf.20120202.11
Khanzada MA, Tanveer M, Maitlo SA, et al. Comparative efficacy of chemical fungicides, plant extracts and bio-control agents against Fusarium solani under laboratory conditions. Pak J Phytopathol. 2016;28(2):133–139.
Parsa S, Sahi ST, Jabbar A, Rehman A, Riaz K, Hannan A. Chemical and biological management of Fusarium oxysporum f. sp melongenae. Pak J Phytopath. 2013;25:155–159.
Yucel S, Elekcioolu YH, Can C, Soout MA, Ozarslandan A. Alternative techniques to control wilts in vegetables. Turkish JAF Sci Tech. 2007;31:47–53.
Singh NI, Devi RT, Devi PP. Effect of fungicides on growth and sporulation of Fusarium solani. Indian Phytopathol. 2000;53(3):327–328.
Dar WA, Beig MA, Ganie SA, Bhat JA, Razvi SM. In vitro study of fungicides and biocontrol agents against Fusarium oxysporum f. sp. pini causing root rot of Western Himalayan fir (Abies pindrow). Sci Res Essay. 2013;8(30):1407–1412.
Yadav SL, Ahir RR, Rathore BS, Yadav SM. Efficacy of different fungicides and organic amendments against basal rot of onion caused by Fusarium oxysporum in vitro. Plant Pathol J. 2014;13(1):56–58.
Jain SK, Khilari KA, Ali MO, Singh RA. Response of Fusarium monoliforme—The causal organism of Bakanae disease of rice against different fungicides. Bioscan. 2014;9(1):413–416.
Copyright (c) 2023 Tooba Ishfaq

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










