Plant Tissue Culture and Formation of Secondary Metabolites - A Review
Abstract
 Abstract Views: 0
Abstract Views: 0
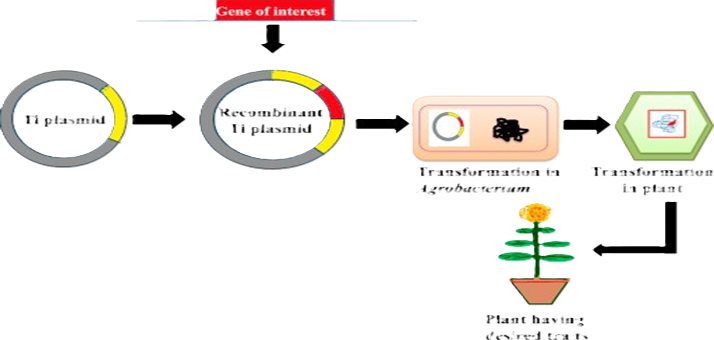
Many copies of a single plant can be grown using the plant tissue culture technology. These copies have the right characteristics to satisfy medical and nutritional demands. Secondary metabolites are purposefully synthesized by using the in vitro technique. These metabolites act as protectors for plants during stressful conditions and offer resistance against different organisms and factors, ultimately helping the plant to survive. With the passage of time, the development of new instruments for the improved synthesis of secondary metabolites via the genetic control of biosynthetic pathways has been aided by the speedy development of recombinant DNA technology. Plants generate a wide range of secondary metabolites that have various biological functions, such as fungicide, herbicide, anti-parasitic, and anti- microbial functions. Nanotechnology has the potential to drastically alter conventional plant growing methods and bring about the synthesis of flavonoids, anthocyanin, and diosgenin by using silver nanoparticles and cadmium oxide nanoparticles (CdONPs). The technique of callus cultures is increasingly utilized to produce secondary metabolites. Hence, the main objective of the current review is to increase the synthesis of secondary metabolites.
Downloads
References
Teng R, Wu Z, Xu S, et al. A novel lateral organ boundary-domain factor CmLBD2 positively regulates pollen development by activating CmACOS5in chrysanthemum morifolium. Plant Cell Physiol. 2021;62(11):1687– 1701. https://doi.org/10.1093/pcp/pca b124
Rahimi S, Bayati M, Kordrostami M, Ghasemi-Soloklui AA. Tissue culture techniques to conserve endangered medicinal plants with antimicrobial and antiviral activities. In: Jha S, Halder M, eds. Medicinal Plants: Biodiversity, Biotechnology and Conservation. Sigapore: Springer; 2023:675–710. https://doi.org/10. 1007/978-981-19-9936 9_24
Bhaskar R, Xavier LSE, Udayakumaran G, Kumar DS, Venkatesh R, Nagella P. Biotic elicitors: a boon for the in vitro production of plant secondary metabolites. Plant Cell Tissue Organ Culture. 2022;149(1-2):7–24. https:// doi.org/10.1007/s11240-021-02131-1
Catarino MD, Silva-Reis R, Chouh A, et al. Applications of antioxidant secondary metabolites of sargassum spp.Marine Drugs. 2023;21(3):e172. https://doi.org/10.33 90/md21030172
Shilpa BM, Rashmi R, Manjula N, Sreekantha A. Bioremediation of heavy metal contaminated sites using phytogenic nanoparticles. In: Shah MP, Roy A,eds. Phytonanotechnology. Singapore: Springer; 2022:227–253. https://doi. org/10.1007/978-981-19-4811-4_11
Riaz U, Hassan A, Fatima M, Aziz H, Rasool M, Murtaza G. Plant secondary metabolites and environmental stress: an overview. In: Roychoudhury A, ed. Biology and Biotechnology of Environmental Stress Tolerance in
Plants. Apple Academic Press; 2023:3–25.
Jakovljević D, Stanković M, Warchoł M, Skrzypek E. Basil (Ocimum L.) cell and organ culture for the secondary metabolites production: a review. Plant Cell Tissue Organ Culture. 2022;149:61–79. https://doi.org/10. 1007/s11240-022-02286-5
Srivastava S, Nalla VK, Singh VP, Prasad R. Optimization of in vitro micropropagation and root establishment through combinatorial approaches for enhanced production of secondary metabolites in the endangered species Decalepis arayalpathra KMA 05 clones. Braz J Bot. 2022;45:869–881. https://doi.org/ 10.1007/s40415-022-00815-2
Machiani MA, Javanmard A, Habibi Machiani R, Sadeghpour A. Arbuscular mycorrhizal fungi and changes in primary and secondary metabolites.Plants. 2022;11(17):e2183. https://doi.org/10. 3390/plants11172183
Akbar MU, Aqeel M, Shah MS, et al. Molecular regulation of antioxidants and secondary metabolites act in conjunction to defend plants against pathogenic infection. S Afr J Bot. 2023;161:247–257. https://doi.org/ 10.1016/j.sajb.2023.08.028
Aware CB, Patil DN, Suryawanshi SS, et al. Natural bioactive products as promising therapeutics: a review of natural product-based drug development. S Afr J Bot. 2022;151:512–528. https://doi.org/ 10.1016/j.sajb.2022.05.028
Wu T, Kerbler SM, Fernie AR, Zhang Y. Plant cell cultures as heterologous bio-factories for secondary metabolite production. Plant Commun. 2021;2(5):e100235 https://doi.org/ 10.1016/j.xplc.2021.100235
Mathew MM, Khatana K, Vats V, et al. Biological approaches integrating algae and bacteria for the degradation of wastewater contaminants—a review. Front Microbiol. 2022;12:e801051. https://doi.org/ 10.3389/fmicb.2021.801051
Devi MK, Manikandan S, Oviyapriya M, et al. Recent advances in biogas production using Agro-Industrial Waste: a comprehensive review outlook of Techno-Economic analysis. Biores Technol. 2022;363:e127871. https://doi.org/10.1016/j.biortech.202 2.127871
Ayele A, Getachew D, Kamaraj M, Suresh A. Phycoremediation of synthetic dyes: an effective and eco- friendly algal technology for the dye abatement. J Chem. 2021;2021:e9923643. https://doi.org/ 10.1155/2021/9923643
Halder M, Sarkar S, Jha S. Elicitation: a biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng Life Sci. 2019;19(12):880–895. https://doi.org/ 10.1002/elsc.201900058
Poupard O, Aıt-Mokhtar A, Dumargue P. Corrosion by chlorides in reinforced concrete: Determination of chloride concentration threshold by impedance spectroscopy. Cement Concrete Res. 2004;34(6):991–1000. https://doi.org/ 10.1016/j.cemconres.2003.11.009
Pang Z, Chen J, Wang T, et al. Linking plant secondary metabolites and plant microbiomes: a review. Front Plant Sci. 2021;12:e621276. https://doi.org/ 10.3389/fpls.2021.621276
Olano C, Lombo F, Mendez C, Salas JA. Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metabolic Eng. 2008;10(5):281–292. https://doi.org/ 10.1016/j.ymben.2008.07.001
Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nat Rev Drug Discov. 2020;19(8):513–532. https://doi.org/ 10.1038/s41573-020-0067-7
Jain C, Khatana S, Vijayvergia R. Bioactivity of secondary metabolites of various plants: a review. Int J Pharm Sci Res. 2019;10(2):494–504.
Naik A, Pandya P, Mankad A. A comprehensive review on phytochemicals and medicinal properties of putranjiva roxburghii wall. Int Assoc Biol Comput Digest. 2023;2(1):234–240. https://doi.org/ 10.56588/iabcd.v2i1.118
Fayaz A, Unnisa G, Faizan M, Ahmed S. Medicinal uses of plant secondary metabolites: a brief review. Ind J Appl Pure Bio. 2023;38(1):170–175.
Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem. 2020;148:80–89. https://doi.org/10.1016/j.plaphy.2020. 01.006
Ruttkay-Nedecky B, Krystofova O, Nejdl L, Adam V. Nanoparticles based on essential metals and their phytotoxicity. J Nanobiotechnol. 2017;15:e33. https://doi.org/ 10.1186/s12951-017-0268-3
Wang P, Lombi E, Zhao F-J, Kopittke PM. Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci. 2016;21(8):699–712. https://doi.org/10.1016/j.tplants.2016. 04.005
Mohammed AE. Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles mediated by Eucalyptus camaldulensis leaf extract. Asian Pac J Trop Biomed. 2015;5(5):382–386. https://doi.org/ 10.1016/S2221-1691(15)30373-7
Joshi NC, Gururani P, Gairola SP. Metal oxide nanoparticles and their nanocomposite-based materials as photocatalysts in the degradation of dyes. Biointerf Res Appl Chem. 2022;12:6557–6579. https://doi.org/ 10.33263/BRIAC125.65576579
Jasim B, Thomas R, Mathew J, Radhakrishnan E. Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharmaceut J. 2017;25(3):443–447. https://doi.org/10.1016/j.jsps.2016.09. 012
García-Sánchez S, Bernales I, Cristobal S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genomics. 2015;16:e341. https://doi. org/10.1186/s12864-015-1530-4
Humbal A, Pathak B. Influence of exogenous elicitors on the production of secondary metabolite in plants: a review (''VSI: Secondary Metabolites''). Plant Stress. 2023:e100166. https://doi.org/10. 1016/j.stress.2023.100166
Neumann K-H, Kumar A, Imani J. Callus cultures. In: Plant Cell and Tissue Culture–A Tool in Biotechnology: Basics and Application. Cham: Springer; 2020:25–59.
Selim MSM, Abdelhamid SA, Mohamed SS. Secondary metabolites and biodiversity of actinomycetes. J Genetic Eng Biotech. 2021;19(1):e72. https://doi.org/10.1186/s43141-021- 00156-9
Srivastava R, Panda R, Chakraborty A. Quantification of nitrogen transformation and leaching response to agronomic management for maize crop under rainfed and irrigated condition. Inviron Pollution. 2020;265:e114866. https://doi.org/ 10.1016/j.envpol.2020.114866
Ptak A, Tahchy A, Skrzypek E, Wójtowicz T, Laurain-Mattar D. Influence of auxins on somatic embryogenesis and alkaloid accumulation in Leucojum aestivum callus. Open Life Sci. 2013;8(6):591– 599. https://doi.org/10.2478/s11535- 013-0160-y
Filová A. Production of secondary metabolities in plant tissue cultures. Res J Agricul Sci. 2014;46(1):236– 245.
Purwianingsih W, Febri S, Kusdianti K. Formation flavonoid secondary metabolites in callus culture of chrysanthemum cinerariefolium as alternative provision medicine. Paper presented at: AIP Conference; 2016; Indonesia. https://doi.org/10.1063/1.4941150
Ibrahim M. Role of endogenous and exogenous hormones in bioactive compounds production in medicinal plants via in vitro culture technique. In: Hano C, ed. Plant Hormones-Recent Advances, New Perspectives and Applications. Intech Opern; 2022:131– 144.
Efferth T. Biotechnology applications of plant callus cultures. Engineering. 2019;5(1):50–59. https://doi.org/ 10.1016/j.eng.2018.11.006
Tiwari R, Rana C. Plant secondary metabolites: a review. Int J Eng Res General Scui. 2015;3(5):661–670.
Sangwan NS, Jadaun JS, Tripathi S, Mishra B, Narnoliya LK, Sangwan RS. Plant metabolic engineering. In: Barh D, Azevedo V, eds. Omics Technologies and Bio-Engineering. Academic Press; 2018:143–175.
Marslin G, Siram K, Maqbool Q, et al. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials. 2018;11(6):e940. https://doi.org/10.3390/ma1106094
Ali A, Mohammad S, Khan MA, et al. Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma tuberculata. Artif Cells Nanomed Biotechnol. 2019;47(1):715–724. https://doi.org/ 10.1080/21691401.2019.1577884
Bourgaud F, Gravot A, Milesi S, Gontier E. Production of plant secondary metabolites: a historical perspective. Plant Sci. 2001;161(5):839–851. https://doi.org/ 10.1016/S0168-9452(01)00490-3
Copyright (c) 2023 Wasiq Ikram, Tooba Sehar, Affifa Atta, Abdul Qadeer Wahla, Syed Muhammad Waqas Gillani; Muhammad Adil Rehman

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










