Evaluating the Antibacterial Activity of Moringa oleifera Leaves Extracts against Pathogenic Bacterial Isolates
Abstract
 Abstract Views: 0
Abstract Views: 0
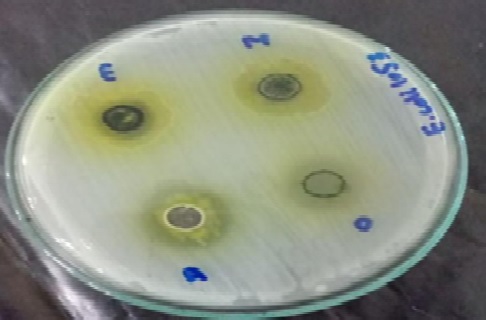
Multi-drug resistant (MDR) bacterial infections pose a major threat to global health. The emergence of antibiotic resistance is due to the overuse and misuse of antibiotics. To overcome this problem, phytochemicals extracted from medicinal plants present an attractive alternative. This study was designed to evaluate the antibacterial and antibiofilm activities of Moringa oleifera leaves extracts against human pathogens. Moringa oleifera leaves were collected and their extracts were prepared in methanol, ethanol, water, and dimethyl sulfoxide solvents. Human pathogenic bacteria were isolated from the urine, sputum, and blood samples of patients from a tertiary care hospital in Lahore. Bacterial isolates were characterized based on their morphological, biochemical, and physiological characteristics. Antibacterial activity of antibiotics was checked through the disc diffusion method. Furthermore, the ability of bacterial strains to form biofilms was observed using qualitative ring test and quantitative microtiter plate assay. Bacterial strains were identified as Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Antibacterial activity of Moringa oleifera leaves extracts, checked by agar well diffusion assay, displayed maximum inhibitory effect (25 mm) in aqueous extract against the strain E2. All of the bacterial strains were found resistant to almost all tested antibiotics, except fosfomycin and amikacin. All bacterial isolates exhibited the potential of biofilm formation. Among all isolates, E2 and E3 bacterial strains appeared as strong slime producers. It was concluded that the significant antibacterial and antibiofilm activity of Moringa oleifera leaves extracts present it as a potential source for novel therapeutic compounds. So, it should be purified and characterized further by using advanced techniques.
Downloads
References
Frei A, Verderosa AD, Elliott AG, Zuegg J, Blaskovich MAT. Metals to combat antimicrobial resistance. Nat Rev Chem. 2023;7(3):202–224. https:// doi.org/10.1038/s41570-023-00463-4
Murray CJ, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. https://doi. org/10.1016/S0140-6736(21)02724-0
Costanzo V, Roviello GN. The potential role of vaccines in preventing antimicrobial resistance (AMR): an update and future perspectives. Vaccines. 2023;11(2):1–21. https:// doi.org/10.3390/vaccines11020333
Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: Causes, consequences, and management. Front Public Health. 2014;2(145):1–8. https://doi.org/10.3389/fpubh.2014.00145
Lv X, Wang L, Mei A, et al. Recent nanotechnologies to overcome the bacterial biofilm matrix barriers. Small. 2023;19(6):1–17. https://doi. org/10.1002/smll.202206220
Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol. 2008;322:107–131. https:// doi.org/10.1007/978-3-540-75418-3_6
Moreno-Camacho CA, Montoya-Torres JR, Jaegler A, Gondran N. Sustainability metrics for real case applications of the supply chain network design problem: a systematic literature review. J Clean Prod. 2019;231:600–618. https://doi.org/10. 1016/j.jclepro.2019.05.278
Neopane P, Nepal HP, Shrestha R, Uehara O, Abiko Y. In vitro biofilm formation by Staphylococcus aureus isolated from wounds of hospital-admitted patients and their association with antimicrobial resistance. Int J Gen Med. 2018;11:25–32. https://doi.org/ 10.2147/IJGM.S153268
Nirwati H, Sinanjung K, Fahrunissa F, et al. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019;13:1–8. https://doi.org/10.1186/s12919-019-0176-7
Reardon, S. WHO warns against “post-antibiotic” era. Nature. 2014;1–2. https://doi.org/10.1038/nature.2014.15135
Mohlala K, Offor U, Monageng E, Takalani NB, Opuwari CS. Overview of the effects of moringa oleifera leaf extract on oxidative stress and male infertility: a review. Appl Sci. 2023;13(7):e4387. https://doi.org/10. 3390/app13074387
Gopalakrishnan L, Doriya K, Kumar DS. Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci Hum Wellness. 2016;5(2):49–56. https://doi.org/10. 1016/j.fshw.2016.04.001
Sales MDC, Costa HB, Fernandes PMB, Ventura JA, Meira DD. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac J Trop Biomed, 2016;6(1): 26–31. https://doi.org/10.1016/j.apjtb.2015.09.026
Ghosh A, Das BK, Roy A, Mandal B, Chandra G. Antibacterial activity of some medicinal plant extracts. J Nat Med. 2008;62(2):259–262. https://doi. org/10.1007/s11418-007-0216-x
Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytother Res, 2015;29(6):796–804. https://doi.org/ 10.1002/ptr.5325
Arif Y, Bajguz A, Hayat S. moringa oleifera extract as a natural plant biostimulant. J Plant Growth Regul. 2023;42:1291–1306. https://doi.org /10.1007/s00344-022-10630-4
Sneath PHA, Mair NS, Sharpe ME, Holt JG. Bergey’s Manual Of Systematic Bacteriology. vol 2. Baltimore, USA; Williams & Wilkins; 1986.
Mahmood KT, Mugal T, Haq, IU. Moringa oleifera: A natural gift-a review. J Pharm Sci. 2010;2(11):775–781.
Mohsenipour Z. The effects of Allium sativum extracts on biofilm formation and activities of six pathogenic bacteria. Jundishapur J Microbiol. 2015;8(8):e48733. https://doi.org/10. 5812/jjm.18971v2
Aliyu AB, Musa AM, Abdullahi MS, Ibrahim H, Oyewale AO. Phytochemical screening and antibacterial activities of Vernonia ambigua, Vernonia blumeoides and Vernonia oocephala (asteraceae). Acta Pol Pharm - Drug Res. 2011;68(1):67–73.
Ali MH, Mustafa, ARA, El-Sheikh AA. Geochemistry and spatial distribution of selected heavy metals in surface soil of Sohag, Egypt: a multivariate statistical and GIS approach. Environ Earth Sci. 2016;75(18):1–17. https://doi.org/ 10.1007/s12665-016-6047-x
Rehman S, Ghauri SM, Sabri AN. Impact of plant extracts and antibiotics on biofilm formation of clinical isolates from otitis media. Jundishapur J Microbiol. 2016;9(1):e29483. https://doi.org/10.5812/jjm.29483
Eladawy M, El-Mowafy M, El-Sokkary MMA, Barwa R. Effects of lysozyme, proteinase k, and cephalosporins on biofilm formation by clinical isolates of pseudomonas aeruginosa. Interdiscip Perspect Infect Dis. 2020;2020:e6156720. https://doi. org/10.1155/2020/6156720
Exner M, Bhattacharya S, Christiansen B, et al. Antibiotic resistance : what is so special about multidrug-resistant Gram-negative bacteria ? antibiotikaresistenz : was ist so besonders an den Gram-negativen. GMS HIC. 2017;12:1–24.
Fotso GW, Kamdem LM, Dube M, et al. Antimicrobial secondary metabolites from the stem barks and leaves of Monotes kerstingii Gilg (Dipterocarpaceae). Fitoterapia. 2019;137:e104239. https://doi.org/10. 1016/j.fitote.2019.104239
Casciaro B, Calcaterra A, Cappiello F, et al. Nigritanine as a new potential antimicrobial aureus-Induced infections. Toxins. 2019;11(9):e511. https://doi.org/10.3390/toxins11090511
Paudyal SP, Kunwar B, Paudel N, Das BD. Isolation and characterization of rhizobia from the root nodule of some cultivated legume crops. Eur J Biol. 2021;11(3):294–306. http://dx.doi.org /10.5281/zenodo.4906255
Anand T, Virmani N, Kumar S, et al. Phage therapy for treatment of virulent Klebsiella pneumoniae infection in a mouse model. J Glob Antimicrob Resist. 2020;21:34–41. https://doi.org/ 10.1016/j.jgar.2019.09.018
Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379–1390. https://doi.org/10.1111/1462-2920.13692
Ervianingsih, Mursyid M, Annisa RN, Zahran I, Langkong J, Kamaruddin I. Antimicrobial activity of Moringa leaf (Moringa oleifera L.) extract against the growth of Staphylococcus epidermidis. Paper presented at: The 1st International Conference of Interdisciplinary Research on Green Environmental Approach for Sustainable Development; August 3–4, 2019; Buton, Indonesia . https://doi. org/10.1088/1755-1315/343/1/012145
Maka G, Shah S, Bano S, Tunio SA. Antibiotic susceptibility profiling of gram-negative bacteria causing upper respiratory tract infections in Hyderabad, Sindh. J Life Bio Sci Res. 2020;1(1):12–15. https://doi.org/10. 38094/jlbsr112
Ghasemian A, Mobarez AM, Peerayeh SN, Abadi ATB, Khodaparast S, Mahmood SS. Expression of adhesin genes and biofilm formation among Klebsiella oxytoca clinical isolates from patients with antibiotic-associated haemorrhagic colitis. J Med Microbiol. 2019;8(7):978–985. https://doi.org/10.1099/jmm.0.000965
Kochan K, Lai E, Richardson Z, et al. Vibrational spectroscopy as a sensitive probe for the chemistry of intra-phase bacterial growth. Sensors. 2020;20(12):e3452. https://doi.org/ 10.3390/s20123452
Copyright (c) 2023 Sadia Batool, Saba Saba, Atia Iqbal, Azka Naveed, Afshan Zia

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










