Isolation and Characterization of Vermamoeba vermiformis from Swimming Pools in Lahore, Pakistan
Abstract
 Abstract Views: 0
Abstract Views: 0
Background. Free-living amoebae (FLA) are common in aquatic environments and their interaction with humans can lead to significant public health risks. Many of these amoebae are opportunistic pathogens, causing infrequent yet severe diseases. Vermamoeba vermiformis, a widely distributed FLA, has been associated with keratitis infection, often in conjunction with Acanthamoeba. Furthermore, V. vermiformis can serve as a host for pathogenic bacteria, such as Legionella pneumophila and Stenotrophomonas maltophilia, amplifying potential health risks. This study aimed to investigate the presence of FLA in three (3) swimming pools situated in Lahore, Pakistan.
Methodology. A total of eighteen (18) water samples were collected from the swimming pools and filtered using 0.45μm cellulose acetate filter papers. The filter papers were carefully placed upside down on non-nutrient agar (NNA) plates seeded with heat-attenuated E. coli. A pure culture of FLA was obtained through repeated subculturing on NNA plates seeded with E. coli, ensuring the results' reliability and validity.
Results. Samples from all three (3) pools exhibited the presence of FLA. The isolated FLA was identified as V. vermiformis based on its morphological appearance under the light microscope, and molecular characterization was performed using the SSU rRNA gene sequence. The trophozoites of V. vermiformis were elongated and cylindrical, with a single pseudopodium, giving them a limax shape. The cysts of V. vermiformis had a double-walled oval and round structure. A clear hyaloplasm was observed at the anterior end of the pseudopodia of actively moving V. vermiformis under a light microscope.
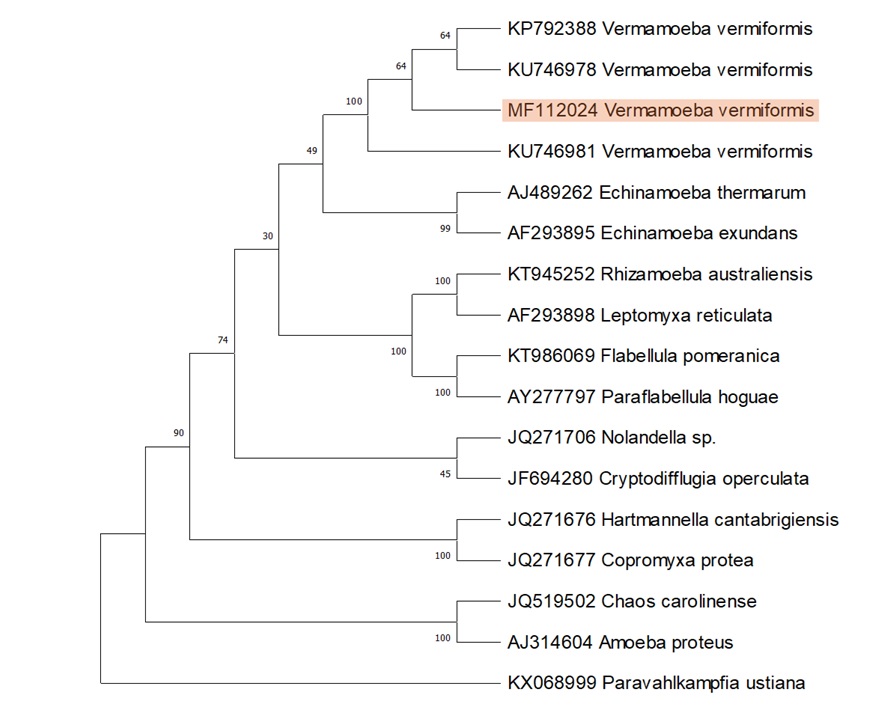
Conclusion. SSU rRNA, gene-based, molecular characterization confirmed isolated FLA as a local isolate of V. vermiformis. Phylogenetic analysis indicated its close homology with Echinamoeba. The presence of V. vermiformis in swimming pool water poses a potential threat to human health, as it is an opportunistic pathogen and a well-known host of different pathogenic bacteria.
Downloads
References
Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. CSH Perspect Med. 2011;1(1):ea006841 https://doi.org/10.1101/cshperspect.a006841
Sun J, He W-T, Wang L, et al. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol Med. 2020;26(5):483–495 https://doi.org/10.1016/j.molmed.2020.02.008
Stockman LJ, Wright CJ, Visvesvara GS, Fields BS, Beach MJ. Prevalence of Acanthamoeba spp. and other free-living amoebae in household water, Ohio, USA—1990–1992. Parasitol Res. 2011;108:621–627. https://doi. org/10.1007/s00436-010-2120-7
Samba-Louaka A, Delafont V, Rodier M-H, Cateau E, Héchard Y. Free-living amoebae and squatters in the wild: ecological and molecular features. FEMS Microbiol Rev. 2019;43(4):415–434. https://doi.org /10.1093/femsre/fuz011
Salazar-Ardiles C, Pérez-Arancibia A, Asserella-Rebollo L, Gómez-Silva B. Presence of free-living acanthamoeba in loa and salado rivers, atacama desert, northern chile. Microorganisms. 2022;10(12):e2315. https://doi.org/10.3390/microorganisms10122315
Leońska-Duniec A, Skotarczak B, Adamska M. Molecular identification of free-living amoebae isolated from artificial water bodies located in Poland. Acta Protozool. 2015;54(1):77–84. https://doi.org/10. 4467/16890027AP.15.006.2193
Salazar-Ardiles C, Asserella-Rebollo L, Andrade DC. Free-living amoebas in extreme environments: the true survival in our planet. Biomed Res Int. 2022;2022:e359883. https://doi.org /10.1155/2022/2359883
Page FC. A New Key To Freshwater And Soil Gymnamoebae: With Instructions For Culture. Freshwater Biological Association; 1988.
Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17(2):413–433. https://doi.org/10.1128/cmr.17.2.413-433.2004
Kuiper MW, Valster RM, Wullings BA, Boonstra H, Smidt H, van der Kooij D. Quantitative detection of the free-living amoeba Hartmannella vermiformis in surface water by using real-time PCR. Appl Environ Microbiol. 2006;72(9):5750–5756. https://doi.org/10.1128/AEM.00085-06
Baquero RA, Reyes-Batlle M, Nicola GG, et al. Presence of potentially pathogenic free-living amoebae strains from well water samples in Guinea-Bissau. Pathog Glob Health. 2014;108(4):206–211. https://doi.org/ 10.1179/2047773214Y.0000000143
Marciano-Cabral F. Free-living amoebae as agents of human infection. J Infect Dis. 2009;199(8):1104–1106, https://doi.org/10.1086/597474
Sente C, Erume J, Naigaga I, et al. Prevalence of pathogenic free-living amoeba and other protozoa in natural and communal piped tap water from Queen Elizabeth protected area, Uganda. Infect Dis Poverty. 2016;5(4):84–97. https://doi.org/ 10.1186/s40249-016-0162-5
Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50(1):1–26. https://doi.org /10.1111/j.1574-695X.2007.00232.x
Marinho BTS, dos Santos DL, dos Santos DL, Rott MB. First report of free-living amoebae in watercourses in southern Brazil: molecular diagnosis and phylogenetic analysis of Vermamoeba vermiformis, Naegleria gruberi, and Acanthamoeba spp. J Water Health. 2023;21(7):972–980. https://doi.org/10.2166/wh.2023.126
Otero-Ruiz A, Gonzalez-Zuñiga LD, Rodriguez-Anaya LZ, Lares-Jiménez LF, Gonzalez-Galaviz JR, Lares-Villa F. Distribution and current state of molecular genetic characterization in pathogenic free-living amoebae. Pathogens. 2022;11(10):e1199. https://doi.org/10.3390/pathogens11101199
Kofman A, Guarner J. Infections caused by free-living amoebae. J Clin Microbiol. 2022;60(1):e00228-21. https://doi.org/10.1128/JCM.00228-21
Smirnov AV, Chao E, Nassonova ES, Cavalier-Smith T. A revised classification of naked lobose amoebae (Amoebozoa: Lobosa). Protist. 2011;162(4):545–570. https://doi.org/10.1016/j.protis.2011.04.004
Delafont V, Rodier M-H, Maisonneuve E, Cateau E. Vermamoeba vermiformis: a free-living amoeba of interest. Microb Ecol. 2018;76:991–1001. https://doi. org/10.1007/s00248-018-1199-8
Azmi WNWN, Hashim F, Suhaili Z, Misbah S, Zakaria NH. Identification of Vermamoeba vermiformis and Tetramitus sp. Isolated from the Gills of Oreochromis sp.(Red Hybrid Tilapia). HAYATI J Biosci. 2023;30(3):404–412. https://doi. org/10.4308/hjb.30.3.404-412
Page FC. Taxonomic criteria for limax amoebae, with descriptions of 3 new species of Hartmannella and 3 of Vahlkampfia. J Protozool. 1967;14(3):499–521. https://doi.org/10.1111/j.1550-7408.1967.tb02036.x
Zettler LAA, Nerad TA, O'Kelly CJ, et al. A molecular reassessment of the leptomyxid amoebae. Protist. 2000;151(3):275–282. https://doi.org/10.1078/1434-4610-00025
Pagnier I, Valles C, Raoult D, La Scola B. Isolation of Vermamoeba vermiformis and associated bacteria in hospital water. Microb Pathog. 2015;80:14–20. https://doi.org/ 10.1016/j.micpath.2015.02.006
Nisar MA, Ross KE, Brown MH, Bentham R, Hinds J, Whiley H. Molecular screening and characterization of Legionella pneumophila associated free-living amoebae in domestic and hospital water systems. Water Res. 2022;226:e119238. https://doi.org/ 10.1016/j.watres.2022.119238
Coşkun KA, Özçelik S, Tutar L, Elaldı N, Tutar Y. Isolation and identification of free-living amoebae from tap water in Sivas, Turkey. Biomed Res Int. 2013;2013:e675145. https://doi.org/10.1155/2013/675145
Park JS. First record of potentially pathogenic amoeba Vermamoeba vermiformis (Lobosea: Gymnamoebia) isolated from a freshwater of Dokdo Island in the East Sea, Korea. Animal Syst Evol Divers. 2016;32(1):1–8. https://doi.org/ 10.5635/ASED.2016.32.1.001
Mahmoudi MR, Rahmati B, Seyedpour SH, Karanis P. Occurrence and molecular characterization of free-living amoeba species (Acanthamoeba, Hartmannella, and Saccamoeba limax) in various surface water resources of Iran. Parasitol Res. 2015;114:4669–4674. https://doi.org /10.1007/s00436-015-4712-8
Dobrowsky PH, Khan S, Cloete TE, Khan W. Molecular detection of Acanthamoeba spp., Naegleria fowleri and Vermamoeba (Hartmannella) vermiformis as vectors for Legionella spp. in untreated and solar pasteurized harvested rainwater. Parasit Vect. 2016;9(1):1–13. https://doi.org/10 .1186/s13071-016-1829-2
Sarink M, van Cappellen W, Tielens A, et al. Vermamoeba vermiformis resides in water-based heater–cooler units and can enhance Mycobacterium chimaera survival after chlorine exposure. J Hosp Infect. 2023;132:73–77. https://doi.org /10.1016/j.jhin.2022.12.011
Reyes-Batlle M, Wagner C, Zamora-Herrera J, et al. Isolation and molecular identification of Vermamoeba vermiformis strains from soil sources in El Hierro Island, Canary Islands, Spain. Curr Microbiol. 2016;73:104–107. https://doi.org/10.1007/s00284-016-1035-7
Milanez GD, Carlos KB, Adao ME, et al. Epidemiology of free-living amoebae infections in Africa: a review. Pathog Glob Health. 2023;117(6):527–534. https://doi.org/ 10.1080/20477724.2022.2160890
Centeno M, Rivera F, Cerva L, et al. Hartmannella vermiformis isolated from the cerebrospinal fluid of a young male patient with meningoencephalitis and bronchopneumonia. Arch Med Res. 1996;27:579–586.
Lorenzo-Morales J, Martínez-Carretero E, Batista N, et al. Early diagnosis of amoebic keratitis due to a mixed infection with Acanthamoeba and Hartmannella. Parasitol Res. 2007;102:167–169. https://doi.org/ 10.1007/s00436-007-0754-x
Kinnear F. Cytopathogenicity of Acanthamoeba, Vahlkampfia and Hartmannella: quantative & qualitative in vitro studies on keratocytes. J Infect. 2003;46(4):228–237. https://doi.org/10.1053/jinf. 2002.1116
Abedkhojasteh H, Niyyati M, Rahimi F, Heidari M, Farnia S, Rezaeian M. First report of Hartmannella keratitis in a cosmetic soft contact lens wearer in Iran. Iran J Parasitol. 2013;8(3):481–485.
Scheid PL, Lâm T-T, Sinsch U, Balczun C. Vermamoeba vermiformis as etiological agent of a painful ulcer close to the eye. Parasitol Res. 2019;118:1999–2004. https://doi.org/ 10.1007/s00436-019-06312-y
Dyková I, Pindová Z, Fiala I, Dvoráková H, Machácková B. Fish-isolated strains of Hartmannella vermiformis Page, 1967: morphology, phylogeny and molecular diagnosis of the species in tissue lesions. Folia Parasitol. 2005;52(4):295–303.
Dyková I, Kostka M, Wortberg F, Nardy E, Pecková H. New data on aetiology of nodular gill disease in rainbow trout, Oncorhynchus mykiss. Folia Parasitol. 2010;57(3):157–163.
Kuiper MW, Wullings BA, Akkermans AD, Beumer RR, Van Der Kooij D. Intracellular proliferation of Legionella pneumophila in Hartmannella vermiformis in aquatic biofilms grown on plasticized polyvinyl chloride. Appl Environ Microbiol. 2004;70(11):6826–6833. https://doi.org/10.1128/AEM.70.11.6826-6833.2004
Ithoi I, Lau Y, Fadzlun AA, Foead A, Neilson R, Nissapatorn V. Detection of free living amoebae, Acanthamoeba and Naegleria, in swimming pools, Malaysia. Trop Biomed. 2010;27(3):566–577.
Smirnov AV, Goodkov AV. An illustrated list of basic morphotypes of Gymnamoebia (Rhizopoda, Lobosea). Protistology. 1999;1(1):20–29.
Lares-Villa F, Hernández-Peña C. Concentration of Naegleria fowleri in natural waters used for recreational purposes in Sonora, Mexico (November 2007–October 2008). Exp Parasitol. 2010;126(1):33–36. https://doi.org/10.1016/j.exppara.2010.04.011
Costa AO, Castro EA, Ferreira GA, Furst C, Crozeta MA, Thomaz‐Soccol V. Characterization of Acanthamoeba isolates from dust of a public hospital in Curitiba, Paraná, Brazil. J Eukaryot Microbiol. 2010;57(1):70–75 https://doi.org/10.1111/j.1550-7408.2009.00453.x
Tsvetkova N, Schild M, Panaiotov S, et al. The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol Res. 2004;92:405–413. https://doi.org/10. 1007/s00436-003-1052-x
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Johnson RO. Notes from the Field: primary amebic meningoencephalitis associated with exposure to swimming pool water supplied by an overland pipe—inyo county, California, 2015. MMWR-Morb Mortal Wkly Rep. 2016;65:e424. http://dx.doi.org/10.15585/mmwr.mm6516a4
Loret J.-F, Greub G. Free-living amoebae: biological by-passes in water treatment. Int J Hyg Environ Health. 2010;213(3):167–175. https:// doi.org/10.1016/j.ijheh.2010.03.004
Gianinazzi C, Schild M, Zumkehr B, et al. Screening of Swiss hot spring resorts for potentially pathogenic free-living amoebae. Exp Parasitol. 2010;126(1):45–53. https://doi.org/ 10.1016/j.exppara.2009.12.008
Zurita-Artaloitia JM, Rivera J, Vinuesa P. Extensive cryptic diversity and ecological associations uncovered among mexican and global collections of Naegleria and Vermamoeba species by 18S Ribosomal DNA, internal transcribed spacer, and cytochrome oxidase subunit I sequence analysis. Microbiol Spect. 2023;11(2):e03795-22. https://doi.org/10.1128/spectrum. 03795-22
Latifi A, Salami M, Kazemirad E, Soleimani M. Isolation and identification of free-living amoeba from the hot springs and beaches of the Caspian Sea. Parasite Epidemiol Cont. 2020;10:e00151. https://doi.org /10.1016/j.parepi.2020.e00151
De Jonckheere JF, Gryseels S, Eddyani M. Knowledge of morphology is still required when identifying new amoeba isolates by molecular techniques. Eur J Protistol. 2012;48(3):178–184. https://doi.org /10.1016/j.ejop.2012.01.009
Fouque E, Héchard Y, Hartemann P, Humeau P, Trouilhé M-C. Sensitivity of Vermamoeba (Hartmannella) vermiformis cysts to conventional disinfectants and protease. J Water Health. 2015;13(2):302–310. https://doi.org/10.2166/wh.2014.154
Susan B. Guide to the methods of study and identification of soil gymnamoebae. Protistology. 2004;3(3):148–190.
Chávez‐Munguía B, Omaña‐Molina M, González‐Lázaro M, González‐Robles A, Bonilla P, Martínez‐Palomo A. Ultrastructural study of encystation and excystation in Acanthamoeba castellanii. J Eukaryot Microbiol. 2005;52(2):153–158. https://doi.org/10.1111/j.1550-7408.2005.04-3273.x
Nazar M, Haghighi A, Taghipour N, et al. Molecular identification of Hartmannella vermiformis and Vannella persistens from man-made recreational water environments, Tehran, Iran. Parasitol Res. 2012;111:835–839. https://doi.org/1 0.1007/s00436-012-2906-x
He Z, Wang L, Ge Y, et al. Both viable and inactivated amoeba spores protect their intracellular bacteria from drinking water disinfection. J Hazard Mater. 2021;417:e126006. https://doi.org/10.1016/j.jhazmat.2021.126006
Swanson M, Hammer B. Legionella pneumophila pathogenesis: a fateful journey from amoebae to macrophages. Annu Rev Microbiol. 2000;54(1):567–613. https://doi.org/ 10.1146/annurev.micro.54.1.567
Carter CJ, Corley EM, Canepa H, Schmalzle SA. Legionnaires’ disease presenting with exanthem; case and review of previously published cases. ID Cases. 2022;27:e01376. https://doi.org/10.1016/j.idcr.2022.e01376
Gomes TS, Vaccaro L, Magnet A, et al. Presence and interaction of free-living amoebae and amoeba-resisting bacteria in water from drinking water treatment plants. Sci Total Env. 2020;719:e137080. https://doi.org/10. 1016/j.scitotenv.2020.137080
Corsaro D, Feroldi V, Saucedo G, Ribas F, Loret JF, Greub G. Novel chlamydiales strains isolated from a water treatment plant. Environ Microbiol. 2009;11(1):188–200. https://doi.org/10.1111/j.1462-2920.2008.01752.x
Corsaro D, Pages GS, Catalan V, Loret J-F, Greub G. Biodiversity of amoebae and amoeba-associated bacteria in water treatment plants. Int J Hyg Environ Health. 2010;213(3):158–166. https://doi.org/ 10.1016/j.ijheh.2010.03.002
Scheid P. Free-living amoebae in rivers and ponds and their multiple role in environmental health. In: Mehlhorn H, Klimpel S, eds. Parasite and Disease Spread by Major Rivers on Earth: Past and Future Perspectives. Springer; 2019:431–444. https://doi.org/10.1007/978-3-030-29061-0_20
Copyright (c) 2024 Muhammad Tariq Zahid, Amber Chayyan, Asmat Ullah, Ayesha Razzaq, Romassa Sajid, Atiqa Ikhlaq, Khajid Ullah Khan, Ghulam Mustafa

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










