Influence of Calcium and Phosphorous Ratio on Hematology and Muscle Proximate Composition of Hypophthalmichthys molitrix Fingerlings
Abstract
 Abstract Views: 0
Abstract Views: 0
Background. Calcium (Ca) and phosphorous (P) are essential minerals for fish growth and development, but Ca absorption from water is limited and its presence also influences P absorption. Therefore, dietary Ca and P are vital for fish to perform physiological activities efficiently.
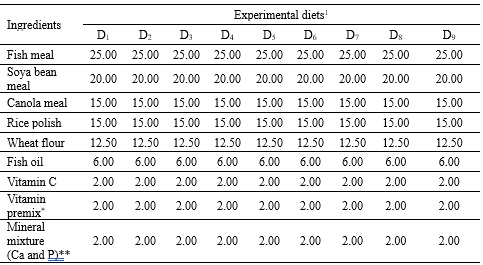
Methodology. This study investigated the interactive effect of Ca and P ratio (Ca:P) on muscle proximate composition and hematology of Hypophthalmichthys molitrix fingerlings (average initial weight = 13.7 ± 0.05 g) for 90 days. Calcium lactate and sodium di-phosphate were used as Ca and P sources. A total of 9 isonitrogenous, isolipidic, and isocaloric diets were formulated by combining 3 Ca levels (0%, 1%, and 2%) with three P levels (0%, 1%, and 2%) and fed to fish twice a day at 5% body weight.
Results. The results showed that moisture and crude protein content in muscles significantly increased (p<0.05) the interactive effect of Ca and P except fat and ash content (p>0.05). Furthermore, the hematological parameters of fingerlings remained unaffected (p>0.05) by the individual supplementation of Ca. However, P supplementation significantly affected the MCV, MHCH, and PLT count. Moreover, the interactive supplementation of Ca and P did not show a significant effect except HCT and MHCH. Platelets count increased at 1% of Ca and 1% of P supplementation, while the RBC count increased at 2% and 1 to 2% (Ca/P). The remaining blood counts did not show considerable variation upon supplementation of Ca/P at different levels.
Conclusion. It was concluded that mineral supplementation showed promising results at 1:2 (Ca/P) level for the optimum performance of H. molitrix.
Downloads
References
Chan CY, Tran N, Dao DC, et al. Fish to 2050 in the ASEAN Region. WorldFish Center and Inernational Food Policy Research Institute; 2017.
Bogard JR, Farook S, Marks GC, et al. Higher fish but lower micronutrient intakes: temporal changes in fish consumption from capture fisheries and aquaculture in Bangladesh. PloS One. 2017;12(4):e0175098. https:// doi.org/10.1371/journal.pone.0175098
Food and Agriculture Organization of the United Staes. The state of world fisheries and aquaculture. FAO Website. https://openknowledge.fao. org/items/11a4abd8-4e09-4bef-9c12-900fb4605a02. Updated 2022. Accessed July 27, 2024.
Cho J, Kim I. Fish meal–nutritive value. J Anim Physiol Anim Nut. 2011;95(6):685–692. https://doi.org /10.1111/j.1439-0396.2010.01109.x
Hardy RW. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquacul Res. 2010;41(5):770–776. https://doi.org/10.1111/j.1365-2109.2009.02349.x
Hardy RW. Farmed fish diet requirements for the next decade and implications for global availability of nutrients. In: Lim CE, Webster CD, Sheng-Lee C, eds. Alternative Protein Sources in Aquaculture Diets. CRC Press; 2023:1–15.
Wu X, Yu Q, Jiang S, Luo Y. Replacement of fishmeal By 60% with soybean meal or peanut meal in diets for juvenile Mangrove Red Snapper (Lutjanus argentimaculatus)(ForsskÃ¥ l, 1775). Oceanog Fisher Open Access J. 2016;1(1):1–7.
Zhao ZX, Song CY, Xie J, et al. Effects of fish meal replacement by soybean peptide on growth performance, digestive enzyme activities, and immune responses of yellow catfish Pelteobagrus fulvidraco. Fisheries Sci. 2016;82:665–673. https://doi.org /10.1007/s12562-016-0996-6
Wan JJ, Shen MF, Tang JQ, et al. Effects of soybean meal processing treatments on growth performance, nutrient digestibility, nitrogen and phosphorus excretion in red swamp crayfish, procambarus clarkii. Aquacul Int. 2017;25(2):543–554. https://doi.org/10.1007/s10499-016-0052-7
Lall SP. The minerals. In: Fish Nutrition. Elsevier; 2022:469–554.
Gopalraaj J, Velayudhannair K, Arockiasamy JP, Radhakrishnan DK. The effect of dietary supplementation of proteases on growth, digestive enzymes, oxidative stress, and intestinal morphology in fishes–a review. Aquacul Int. 2024;32(1):745–765. https://doi.org/10.1007/s10499-023-01191-8
Hossain M, Yoshimatsu T. Dietary calcium requirement in fishes. Aquacu Nutr. 2014;20(1):1–11. https://doi.org/ 10.1111/anu.12135
Council NR. Nutrient Requirements of Fish and Shrimp. National Academies Press; 2011.
Kandeepan C. Dietary calcium requirement of Oreochromis mossambicus. Int J Curr Microbiol App Sci. 2013;2:89–97.
Liang H, Mi H, Ji K, Ge X, Re M, Xie J. Effects of dietary calcium levels on growth performance, blood biochemistry and whole body composition in juvenile bighead carp (Aristichthys nobilis). Turk J Fisher Aquat Sci. 2018;18(4):623–631.
Shiau SY, Tseng HC. Dietary calcium requirements of juvenile tilapia, Oreochromis niloticus× O. aureus, reared in fresh water. Aquacul Nutr. 2007;13(4):298–303. https://doi.org /10.1111/j.1365-2095.2007.00481.x
Robinson EH, Rawles SD, Brown PB, Yette HE, Greene LW. Dietary calcium requirement of channel catfish Ictalurus punctatus, reared in calcium-free water. Aquaculture. 1986;53(3-4):263–270. https://doi.org /10.1016/0044-8486(86)90356-X
Hossain F. Essentiality of dietary calcium supplement in redlip mullet Liza haematocheila. Aquacul Nutr. 2000;6(1):33–38.
Chavez-Sanchez C, Martinez-Palacios CA, Martinez-Perez G, Ross LG. Phosphorus and calcium requirements in the diet of the American cichlid Cichlasoma urophthalmus (Gunther). Aquacul Nutr. 2000;6(1):1–10.
Brody T. Nutritional biochemistry. Elsevier; 1998.
Lall S. The minerals. Fish nutrition. Elsevier academic press, San Diego, CA, USA. 2002:259–308.
Yang Q, Liang H, Maulu S, et al. Dietary phosphorus affects growth, glucolipid metabolism, antioxidant activity and immune status of juvenile blunt snout bream (Megalobrama amblycephala). Anim Feed Sci Technol. 2021;274:e114896. https://doi.org/10.1016/j.anifeedsci.2021.114896
Adeshina I, Akpoilih BU, Udom BF, Adeniyi OV, Abdel-Tawwab M. Interactive effects of dietary phosphorus and microbial phytase on growth performance, intestinal morphometry, and welfare of Nile tilapia (Oreochromis niloticus) fed on low-fishmeal diets. Aquaculture. 2023;563:e738995. https://doi.org /10.1016/j.aquaculture.2022.738995
Fontagné S, Silva N, Bazin D, et al. Effects of dietary phosphorus and calcium level on growth and skeletal development in rainbow trout (Oncorhynchus mykiss) fry. Aquaculture. 2009;297(1-4):141–150. https://doi.org/10.1016/j.aquaculture.2009.09.022
Shen HM, Chen XR, Chen WY, et al. Influence of dietary phosphorus levels on growth, body composition, metabolic response and antioxidant capacity of juvenile snakehead (C hanna argus× C hanna maculata). Aquacul Nutr. 2017;23(4):662–670. https://doi.org/10.1111/anu.12433
Zafar N, Khan M. Determination of dietary phosphorus requirement of stinging catfish Heteropneustes fossilis based on feed conversion, growth, vertebrae phosphorus, whole body phosphorus, hematology and antioxidant status. Aquacul Nutr. 2018;24(5):1577–1586. https://doi. org/10.1111/anu.12794
Jobling M. National Research Council (NRC): nutrient requirements of fish and shrimp. Aquacult Int. 20:601–602. https://doi.org/10.1007/s10499-011-9480-6
Laining A, Ishikawa M, Kyaw K, et al. Dietary calcium/phosphorus ratio influences the efficacy of microbial phytase on growth, mineral digestibility and vertebral mineralization in juvenile tiger puffer, Takifugu rubripes. Aquacul Nutr. 2011;17(3):267–277. https://doi.org /10.1111/j.1365-2095.2009.00749.x
McDowell LR. Minerals in Human and Animal Nutrition. San Diego: Academic; 1992.
Sun Y, Chen M, Kong C, Tang H, Gan L, Zhang M. Enclosure experiment of effects of dietary phosphorus level on water quality, phosphorus budget, and plankton composition in intensive culture of crucian carp. Aquacul Int. 2017;25:1145–1158. https://doi.org /10.1007/s10499-016-0103-0
Ye CX, Liu YJ, Tian LX, et al. Effect of dietary calcium and phosphorus on growth, feed efficiency, mineral content and body composition of juvenile grouper, Epinephelus coioides. Aquaculture. 2006;255(1-4):263–271. https://doi.org/10.1016 /j.aquaculture.2005.12.028
Global Invasive Species Database. Hypophthalmichthys molitrix https://iucngisd.org/gisd/speciesname/Hypophthalmichthys+molitrix. ISSG Website. Updated 2006. Accessed July 27, 2024.
Gui P, Zhang L, Hong H, Feng L, Luo Y. Gel properties of silver carp (Hypophthalmichthys molitrix) and chicken mixture gels as affected by setting temperatures. Int J Food Prop. 2018;21(1):2250–2264. https://doi.org /10.1080/10942912.2018.1508155
Birdsall B. Factors Related to Occupancy and Detection and Population Demographics of Adult Bighead Carp and Silver Carp in the Lower Red River Catchment [dissertation]. Auburn University; 2023.
Peres H, Costas B, Perez‐Jimenez A, Guerreiro I, Oliva‐Teles A. Reference values for selected hematological and serum biochemical parameters of S enegalese sole (S olea senegalensis Kaup, 1858) juveniles under intensive aquaculture conditions. J Appl Ichthyol. 2015;31(1):65–71. https://doi.org/10.1111/jai.12641
Harikrishnan R, Devi G, van Doan H, et al. Study on antioxidant potential, immunological response, and inflammatory cytokines induction of glycyrrhizic acid (GA) in silver carp against vibriosis. Fish Shellfish Immunol. 2021;119:193–208. https:// doi.org/10.1016/j.fsi.2021.09.040
Fatima M, Afzal M, Shah SZH. Effect of dietary oxidized oil and vitamin E on growth performance, lipid peroxidation and fatty acid profile of Labeo rohita fingerlings. Aquacul Nutr. 2019;25(2):281–291. https://doi.org/10.1111/anu.12851
Akram Z, Fatima M, Shah SZH, et al. Dietary zinc requirement of Labeo rohita juveniles fed practical diets. J Appl Anim Res. 2019;47(1):223–229. https://doi.org/10.1080/09712119.2019.1613238
Khajepour F, Hosseini SA. Calcium and phosphorus status in juvenile Beluga (Huso huso) fed citric acid‐supplemented diets. Aquacul Res. 2012;43(3):407–411. https://doi.org /10.1111/j.1365-2109.2011.02843.x
Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists. Association of Official Analytical Chemists; 1990.
AOAC International. AOAC Research institute’s performance tested methods℠ (PTM) program. https://www.aoac.org/scientific-solutions/research-institute-ptm/. Accessed July 27, 2024.
Steel RGD, Torrie JH. Principles and Procedures of Statistics. McGraw-Hill Book Company; 1960.
Yang SD, Lin TS, Liu FG, Liou CH. Influence of dietary phosphorus levels on growth, metabolic response and body composition of juvenile silver perch (Bidyanus bidyanus). Aquaculture. 2006;253(1-4):592–601. https://doi.org/10.1016/j.aquaculture.2005.09.002
Koko GK, Sarker PK, Proulx É, Vandenberg GW. Effects of alternating feeding regimes with varying dietary phosphorus levels on growth, mineralization, phosphorus retention and loading of large rainbow trout (Oncorhynchus mykiss). Aquat Living Resour. 2010;23(3):277–284. https://doi.org/10.1051/alr/2010032
Lei Y, Sun Y, Wang X, et al. Effect of dietary phosphorus on growth performance, body composition, antioxidant activities and lipid metabolism of juvenile Chinese mitten crab (Eriocheir sinensis). Aquaculture. 2021;531:e735856. https://doi.org /10.1016/j.aquaculture.2020.735856
Ishtiaq S, Fatima M, Shah SZH, Khan N, Bilal M, Nisa S. Optimization of calcium and phosphorous ratio in the practical diet of Hypophthalmichthys molitrix. Pak J Zool. 2023;55(6):e2639. https://dx.doi.org/ 10.17582/journal.pjz/20220515090536
Nakamura Y, Yamada J. Effects of dietary calcium levels, Ca/P ratios, and calcium components on the calcium absorption rate in carp. Bull Fish Sci Hokkaido Univ. 1980;31(4):277–282.
Zhou QC, Liu YJ, Mai KS, Tian LX. Effect of dietary phosphorus levels on growth, body composition, muscle and bone mineral concentrations for orange‐spotted grouper Epinephelus coioides reared in floating cages. J World Aquacul Soc. 2004;35(4):427–435. https://doi.org/10.1111/j.1749-7345.2004.tb00107.x
Tayyaba M, Fatima M, Shah SZH, et al. Dietary phosphorus requirement of silver Carp (Hypophthalmichthys molitrix) fingerlings. J Anim Physiol Anim Nutr. 2024;108(1):27–35. https://doi.org/10.1111/jpn.13863
Tan B, Mai K, Liufu Z. Response of juvenile abalone, Haliotis discus hannai, to dietary calcium, phosphorus and calcium/phosphorus ratio. Aquaculture. 2001;198(1-2):141–158. https://doi.org/10.1016/S0044-8486(00)00595-0
Nwanna L, Oni O. Determination of optimum calcium and phosphorous ratio for the production of African catfish Clarias gariepinus (Burchell, 1822). J Appl Sci Environl Manag. 2018;22(5):689–692. https://doi.org/ 10.4314/jasem.v22i5.12
Roy PK, Lall SP. Dietary phosphorus requirement of juvenile haddock (Melanogrammus aeglefinus L.). Aquaculture. 2003;221(1-4):451–468. https://doi.org/10.1016/S0044-8486(03)00065-6
Robinson EH, LaBomascus D, Brown PB, Linton TL. Dietary calcium and phosphorus requirements of Oreochromis aureus reared in calcium-free water. Aquaculture. 1987;64(4):267–276. https://doi.org /10.1016/0044-8486(87)90189-X
Al-Jubawi E, AL-humairi K. Study of the hematological and some biochemical characteristics in common carp (Cyprinus carpio L.) fish feeding on fermented rations with varying concentrations growth stimulator (Bio boost aqua). Int J Veter Res. 2024;4(1):11–24.
Liang JJ, Liu YJ, Tian LX, Yang HJ, Liang GY. Dietary available phosphorus requirement of juvenile grass carp (Ctenopharyngodon idella). Aquacul Nutr. 2012;18(2):181–188. https://doi.org/10.1111/j.1365-2095.2011.00887.x
Mustafa IA, Omar SS. The effects of dietary organic selenium on growth, body composition and hematological parameters of common Carp (Cyprinus carpio) reared in recirculating aquaculture system. Cellul Molecul Biol. 2024;70(1):87–93. https://doi. org/10.14715/cmb/2024.70.1.12
Copyright (c) 2024 Mahnoor Arshad, Mahroze Fatima, Ayesha Khizar, Haseeba Ishtiaq, Muhammad Adnan Ali, Muhammad Omer Gulzar

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










