Screening Enterococcus Isolates for Antimicrobial and In Vitro Antitumor Activity against Colorectal Carcinoma
Abstract
 Abstract Views: 0
Abstract Views: 0
Background. Enterococci are a part of the natural intestinal flora of humans and animals and play an important role in keeping the microbial balance in the gut. Many species of Enterococci are also used as probiotics that produce vitamins, stimulate the immune response, and maintain the integrity of the gut. The use of dietary supplements to reinforce some gut flora components is a current aspect of functional food sciences to treat various diseases.
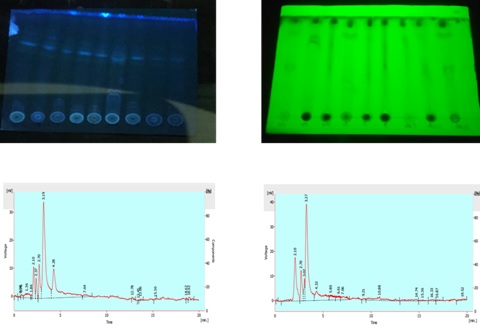
Methodology. In the current study, 21 Enterococcus strains were isolated and identified morphologically, biochemically, and physiologically. The strains were analyzed for their metabolomics potential by using thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC) analyses, while the disc diffusion method was employed to assess their antibacterial potential against the known pathogens. The in vitro antitumor activity was determined against HCT 116 colorectal carcinoma (CRC) cell lines at different concentrations including 12 mg/ml, 25 mg/ml, 50 mg/ml, and 100 mg/ml.
Results. Out of the 21 strains, 9 showed antimicrobial activity against Pseudomonas, Enterobacter, Klebsiella, and Staphylococcus. Several strains showed sensitivity against certain antibiotics, such as amoxicillin, norfloxacin, streptomycin, vancomycin, and nalidixic acid. The crude extracts of the isolates also showed high cytotoxicity against Artemia salina and significant antitumor activity against HCT 116 colorectal carcinoma CRC cell lines. The crude extracts of these Enterococcus strains exhibited the presence of a variety of bioactive metabolites by using TLC and HPLC analysis.
Conclusion. The study revealed that the antimicrobial compounds produced by these bioactive Enterococcus strains can be used against Pseudomonas, Enterobacter, Klebsiella, and Staphylococcus. Moreover, these strains can be investigated as potential probiotic agents to treat colorectal cancer because of their significant in vitro antitumor activity against CRC.
Downloads
References
Kajihara T, Nakamura S, Iwanaga N, et al. Clinical characteristics and risk factors of enterococcal infections in Nagasaki, Japan: a retrospective study. BMC Infect Dis. 2015;15:e426. https://doi.org/10.1186/s12879-015-1175-6
Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009;155(6):1749–1757. https://doi. org/10.1099/mic.0.026385-0
Hashem YA, Yassin AS, Amin MA. Molecular characterization of Enterococcus spp. clinical isolates from Cairo, Egypt. Indian J Med Microbiol. 2015;33:80–86. https://doi. org/10.4103/0255-0857.148836
Hanchi H, Mottawea W, Sebei K, Hammami R. The genus enterococcus: between probiotic potential and safety concerns-an update. Front Microbiol. 2018;9:e1791. https://doi.org/10.3389/ fmicb.2018.01791
Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11(5):4745–4767. https://doi.org/10.3390/ijerph110504745
Nami Y, Bakhshayesh RV, Jalaly HM, Lotfi H, Eslami S, Hejazi MA. Probiotic properties of enterococcus isolated from artisanal dairy products. Front Microbiol. 2019;10:e300. https://doi.org/10.3389/fmicb.2019.00300
Carmen C, Óscar R, Pilar C-M, Marta P, Jorge B-V. Preliminary characterization of bacteriocins from Lactococcus lactis, enterococcus faecium and Enterococcus mundtii strains isolated from turbot (Psetta maxima). Food Res. Int. 2006;39(3):356–364. https://doi.org/ 10.1016/j.foodres.2005.08.008
Gomez J, Vallejo M, Parada RB, Marguet ER, Bellomio A, de Carvalho KG. Exploring the bioactive potential of Enterococcus mundtii TW278: synthesis and utilization of biomolecules in yogurt production. Food Bioscience. 2024;61:e104760. https:// doi.org/10.1016/j.fbio.2024.104760
Rabbani KM, Shafiei R. Traditional yogurt as a source of lactobacilli and other lactic acid bacteria in Iran. In: Shah NP, ed. Yogurt in Health and Disease Prevention. Academic press. 2017;285–294. https://doi.org/10. 1016/B978-0-12-805134-4.00016-X
Rakib MR, Kabir A, Amanullah SM. Starter cultures used in the production of probiotic dairy products and their potential applications: a review. Chem Biomolecul Eng. 2017;2(2):83–89. http://dx.doi.org/10.11648/j.cbe.20170202.12
Kim J, Lee HK. Potential role of the gut microbiome in colorectal cancer progression. Front Immunol. 2022;12:e807648. https://doi.org/10. 3389/fimmu.2021.807648
Śliżewska K, Markowiak-Kopeć P, Śliżewska W. The role of probiotics in cancer prevention. Cancers. 2020;13(1):e20. https://doi.org/10. 3390/cancers13010020
Raheem A, Liang L, Zhang G, Cui S. Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front Immunol. 2021;12:e616713. https:// doi.org/10.3389/fimmu.2021.616713
Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15(3):317–328. https://doi.org/10.1016/j.chom.2014.02.007
Santacroce L, Charitos IA, Bottalico L. A successful history: probiotics and their potential as antimicrobials. Expert Rev Anti Infect Ther. 2019;17(8):635–645. https://doi.org/ 10.1080/14787210.2019.1645597
Anuradha R. High-Throughput screening (HTS) technology. In: Offermanns S, Rosenthal W, eds. Encyclopedia of Molecular Pharmacology. Springer; 2022;787–799. https://doi.org/10.1007/978-3-030-57401-7_73
Hassan A, Ali B, Sajid I. Antimicrobial screening and metabolic fingerprinting of soil bacilli against urinary tract infections (utis) causing E. coli. Sci. Int. 2014;26(4):1569–76.
Nawaz S, Fatima A, Saleem M, Sajid I. Exploring the antimicrobials production potential of actinobacteria isolated from caves at Bahadurkhel Karak, Pakistan: antimicrobial activities of cave actinobacteria against XDR Salmonella. Proceedings of the Pakistan Academy of Sciences: B. Life Environ Sci. 2023;60(1):101–112. https://doi.org/10.53560/PPASB(60-1)785
Meijs J, Pors D, Vlieland TPV, Huizinga TW, Schouffoer AA. Translation, cross-cultural adaptation, and validation of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument (SCTC GIT) 2.0 into Dutch. Clin Exp Rheumatol. 2014;32(86):eS-41-8.
McQuade1 R, Bornstein J, Nurgali K. Anti-colorectal cancer chemotherapy-induced diarrhoea: current treatments and side-effects. Int J Cli. Med. 2014;5(7):393–406. http://dx.doi.org /10.4236/ijcm.2014.57054
Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol. 2011;45:S120–S127. https://doi.org /10.1097/mcg.0b013e31822fecfe
Chaudhary A, Prajapati N, Prajapati A, et al. Postbiotic emissaries: a comprehensive review on the bioprospecting and production of bioactive compounds by Enterococcus species. Int J Food Sci Technol. 2024;59(10):6769–6782. https://doi.org/10.1111/ijfs.17431
Line JE, Svetoch EA, Eruslanov BV, et al. Isolation and purification of enterocin E-760 with broad antimicrobial activity against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2008;52(3):1094–1100. https://doi.org /10.1128/aac.01569-06
De Kwaadsteniet M, Todorov SD, Knoetze H, Dicks LM. Characterization of a 3944 Da bacteriocin, produced by Enterococcus mundtii ST15, with activity against Gram-positive and Gram-negative bacteria. Int J Food Microbiol. 2005;105(3):433–444. https://doi.org /10.1016/j.ijfoodmicro.2005.03.021
Zhang YJ, Li S, Gan RY, Zhou T, Xu DP, Li HB. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16(4):7493–7519. https://doi.org/10.3390/ijms16047493
Imran S, Yao CBFF, Khaled S, Shahida H, Hartmut L. Antifungal and antibacterial activities of indigenous Streptomyces isolates from saline farmlands: prescreening, ribotyping and metabolic diversity. WJMB. 2009;25(4):601–610. https://doi.org /10.1007/s11274-008-9928-7
Albaayit SFA, Maharjan R, Abdullah R, Noor MHM. Anti-Enterococcus Faecalis, Cytotoxicity, Phytotoxicity, and Anticancer Studies on Clausena excavata Burum. f. (Rutaceae) Leaves. Biomed Res Int. 2021;2021:e3123476. https://doi.org/10.1155/2021/3123476
Copyright (c) 2024 Ayesha Siddiqa, Ashba Hassan, Shahid Nawaz, Imran Sajid

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










