Molecular Mechanisms Underlying Anticancer and Anti-Inflammatory Activities of Oridonin in Oral Squamous Cell Carcinoma
Abstract
 Abstract Views: 0
Abstract Views: 0
Background. Oral squamous cell carcinoma (OSCC) constitutes one of the most common pathological forms of oral cancers. Oridonin is an ent-kaurane diterpenoid compound isolated from Rabdosia rubescens. Recently, the anticancer potential of Oridonin has been extensively studied in breast, osteosarcoma, myeloma, neuroblastoma, lymphoma, pancreatic, colon, leukemia, and esophageal cancers. The anticancer potential of Oridonin is largely unexplored in OSCC.
Method. This study aimed to provide insights into the multifunctional anticancer activities of Oridonin in OSCC. We carried out an extensive and critical literature survey on research related to the importance of medicinal plants in various cancers, role of Oridonin as potential anticancer agents in OSCC up to 2025 using keywords apoptotic proteins, antitumor activities, cell cycle arrest, diterpenoid, inflammasomes, Notch signaling pathway, natural products, Oridonin, oral squamous cell carcinoma, and oral cancer treatment.
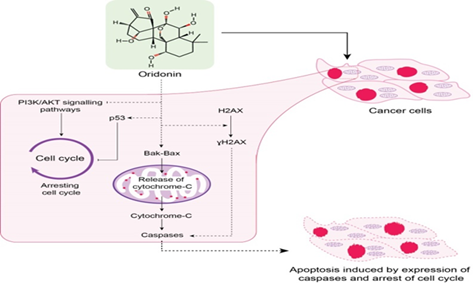
Results. Oridonin induces cell apoptosis in oral cancer cells (OCC) by regulating mitochondrial and ROS-mediated JNK/p38 MAPK, acting as cell cycle blocker at the G2/M phase pathways, and increasing the expression of γH2AX. Oridonin plays an essential role in OSCC tumorigenesis by inhibiting the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway. Blocking Notch signaling dysregulation and specific inhibition of NLRP3 inflammasome are the other cellular mechanism by which Oridonin can exhibit its antitumor activities.
Conclusion. Oridonin can serve as a potential anticancer drug in OSCC due to its involvement in multiple cellular signaling pathways.
Downloads
References
Golburean O, Uncuta D, Manrikyan G, et al. Exploring dental students' knowledge on oral cancer prevention: a cross-sectional study in Moldova, Armenia, and Belarus. BMC Oral Health. 2025;25(1):e81. https://doi.org /10.1186/s12903-025-05459-8
Vinay V, Jodalli P, Chavan MS, et al. Artificial intelligence in oral cancer: a comprehensive scoping review of diagnostic and prognostic applications. Diagnostics. 2025;15(3):e280. https:// doi.org/10.3390/diagnostics15030280
Qian Y, Tang L, Yao J, et al. Pembrolizumab with chemotherapy for patients with recurrent or metastatic nasal cavity and paranasal sinus squamous cell carcinoma: A prospective phase ll study. Clinic Cancer Res. 2025:OF1–OF8. https://doi.org/10.1158/1078-0432.CCR-24-4148
Yun H-M, Kim B, Kim SH, Kwon S-H, Park K-R. Xanol promotes apoptosis and autophagy and inhibits necroptosis and metastasis via the inhibition of AKT signaling in human oral squamous cell carcinoma. Cells. 2023;12(13):e1768. https://doi.org/ 10.3390/cells12131768
Shaharudin NS, Singh GKS, Kek TL, Sultan S. Targeting signaling pathways with andrographolide in cancer therapy. Mol Clinic Oncol. 2024;21(5):e81. https://doi.org/10. 3892/mco.2024.2779
Huang J, Fu X, Qiu F, et al. Discovery of a natural ent-kaurene diterpenoid oridonin as an E3 ligase recruiter for PROTACs. This study elucidates the structural attributes of oridonin, an ent-kaurene diterpenoid, highlighting its capacity to function as an E3 ligase recruiter in PROTACs (Proteolysis Targeting Chimeras). by leveraging oridonin's unique structure, the research demonstrates its role in facilitating the degradation of oncogenic proteins, such as BRD4 and EGFR, thereby showcasing its potential in targeted cancer therapies. J Am Chem Soc. 2024;147(2):1920–1937. https://doi.org/10.1021/jacs. 4c14650
Yasuda S, Horinaka M, Iizumi Y, Goi W, Sukeno M, Sakai T. Oridonin inhibits SASP by blocking p38 and NF-κB pathways in senescent cells. Biochem Biophy Res Commun. 2022;590:55–62. https://doi.org/10. 1016/j.bbrc.2021.12.098
Cheng Y, Chen J, Shi Y, Fang X, Tang Z. MAPK signaling pathway in oral squamous cell carcinoma: biological function and targeted therapy. Cancers. 2022;14(19):e4625. https:// doi.org/10.3390/cancers14194625
Wang H, Zhu L, Feng X, Zhang H, Luo Q, Chen F. Oridonin induces G2/M cell cycle arrest and apoptosis in human oral squamous cell carcinoma. This study highlights that Oridonin acts as a cell cycle blocker by causing G2/M phase arrest and induces apoptosis in human oral squamous cell carcinoma (OSCC). The anticancer effects of oridonin are mediated through the upregulation of p53 and p21, activation of the p38 MAPK pathway, downregulation of Bcl-2, and inhibition of NF-κB signaling based critical pathways involved in cell cycle regulation and apoptosis. Eur J Pharmacol. 2017;815:282–289. https://doi.org/10.1016/j.ejphar.2017.09.021
Yang J, Zhao X, Tang M, et al. The role of ROS and subsequent DNA-damage response in PUMA-induced apoptosis of ovarian cancer cells. Oncotarget. 2017;8(14):23492–23506. https://doi.org/10.18632/oncotarget.15626
Yang J, Ren X, Zhang L, Li Y, Cheng B, Xia J. Oridonin inhibits oral cancer growth and PI3K/Akt signaling pathway. This study demostrates that Oridonin serves as a potent PI3K/Akt pathway inhibitor, effectively suppressing tumorigenesis in oral squamous cell carcinoma (OSCC). It exerts its anticancer activity by downregulating the phosphorylation of PI3K and Akt, leading to G2/M phase cell cycle arrest and induction of apoptosis in OSCC cells. These findings highlight the potential of oridonin as a targeted therapeutic agent in oral cancer through modulation of the PI3K/Akt signaling axis. Biomed Pharmacother. 2018;100:226–232. https://doi.org/10.1016/j.biopha.2018.02.011
Oh H-N, Seo J-H, Lee M-H, et al. Oridonin induces apoptosis in oral squamous cell carcinoma probably through the generation of reactive oxygen species and the p38/JNK MAPK pathway. This study explored the anticancer effects of oridonin on oral squamous cell carcinoma (OSCC) cells. The authors demonstrated that oridonin induces apoptosis through the generation of reactive oxygen species (ROS) and subsequent activation of the p38 and JNK branches of the MAPK signaling pathway. This mechanistic insight underscores the role of MAPK cascades in mediating oridonin's cytotoxic effects on oral cancer cells. The findings support oridonin’s potential as a therapeutic agent by elucidating its apoptotic pathways in OSCC, especially in relation to oxidative stress and MAPK pathway involvement. Int J Oncol. 2018;52(5):1749–1759. https://doi.org/10.3892/ijo.2018.4319
Xia S, Zhang X, Li C, Guan H. Oridonin inhibits breast cancer growth and metastasis through blocking the Notch signaling. Saudi Pharm J. 2017;25(4):638–643. https://doi.org/ 10.1016/j.jsps.2017.04.037
He H, Jiang H, Chen Y, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9(1):e2550. https://doi.org/10.1038/s41467-018-04947-6
Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):e128. https://doi.org/10.1038/s41419-019-1413-8
Zhao Z, Chen Y. Oridonin, a promising antitumor natural product in the chemotherapy of hematological malignancies. Current Pharm Biotechnol. 2014;15(11):1083–1092.
Zhou G-B, Chen S-J, Wang Z-Y, Chen Z. Back to the future of oridonin: again, compound from medicinal herb shows potent antileukemia efficacies in vitro and in vivo. Cell Res. 2007;17(4):274–276. https://doi.org/10.1038/cr.2007.21
Fujita E, Nagao Y, Node M, Kaneko K, Nakazawa S, Kuroda H. Antitumor activity of the Isodon diterpenoids: structural requirements for the activity. Experientia. 1976;32(2):203–206. https://doi.org/10.1007/BF01937766
Li X, Zhang C-T, Ma W, Xie X, Huang Q. Oridonin: a review of its pharmacology, pharmacokinetics and toxicity. Front Pharm. 2021;12:e645824. https://doi.org/10.3389/fphar.2021.645824
Wu Q-X, Yuan S-X, Ren C-M, et al. Oridonin upregulates PTEN through activating p38 MAPK and inhibits proliferation in human colon cancer cells. Oncol Rep. 2016;35(6):3341–3348. https://doi.org/10.3892/or.2016.4735
Bu H-Q, Shen F, Cui J. The inhibitory effect of oridonin on colon cancer was mediated by deactivation of TGF-β1/Smads-PAI-1 signaling pathway in vitro and vivo. OncoTargets Ther. 2019;12:7467–7476.
Lou H, Zhang X, Gao L, et al. In vitro and in vivo antitumor activity of oridonin nanosuspension. Int J Pharm. 2009;379(1):181–186. https://doi.org/10.1016/j.ijpharm.2009.06.022
Wang S, Zhong Z, Wan J, et al. Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am J Chin Med. 2013;41(1):177–196. https://doi.org/10.1142/S0192415X13500134
Ye LH, Li WJ, Jiang XQ, et al. Study on the autophagy of prostate cancer PC‐3 cells induced by oridonin. Anatom Record Adv Integ Anatomy Evol Biol. 2012;295(3):417–422. https://doi.org/10.1002/ar.21528
Ye Y-c, Wang H-j, Xu L, et al. Oridonin induces apoptosis and autophagy in murine fibrosarcoma L929 cells partly via NO-ERK-p53 positive-feedback loop signaling pathway. Acta Pharmcol Sin. 2012;33(8):1055–1061. https://doi.org/10.1038/aps.2012.53
Yu Y, Fan SM, Song JK, Tashiro S-I, Onodera S, Ikejima T. Hydroxyl radical (· OH) played a pivotal role in oridonin-induced apoptosis and autophagy in human epidermoid carcinoma A431 cells. Biol Pharm Bull. 2012;35(12):2148–2159. https://doi.org/10.1248/bpb.b12-00405
Zang L, He H, Ye Y, et al. Nitric oxide augments oridonin-induced efferocytosis by human histocytic lymphoma U937 cells via autophagy and the NF-κB-COX-2-IL-1β pathway. Free Rad Res. 2012;46(10):1207–1219. https://doi.org/10.3109/10715762.2012.700515
Li D, Wu L-J, Tashiro S-I, Onodera S, Ikejima T. Oridonin induces human epidermoid carcinoma A431 cell apoptosis through tyrosine kinase and mitochondrial pathway. J Asian Nat Products Res. 2008;10(1):77–87. https://doi.org/10.1080/10286020701273866
Zhu M, Hong D, Bao Y, Wang C, Pan W. Oridonin induces the apoptosis of metastatic hepatocellular carcinoma cells via a mitochondrial pathway. Oncol Lett. 2013;6(5):1502–1506. https://doi.org/10.3892/ol.2013.1541
Liu Y-Q, Mu Z-Q, You S, Tashiro S-I, Onodera S, Ikejima T. Fas/FasL signaling allows extracelluar-signal regulated kinase to regulate cytochrome c release in oridonin-induced apoptotic U937 cells. Biol Pharm Bullet. 2006;29(9):1873–1879. https://doi.org/10.1248/bpb.29.1873
Zheng M, Zhu Z, Zhao Y, Yao D, Wu M, Sun G. Oridonin promotes G2/M arrest in A549 cells by facilitating ATM activation. Molecul Med Rep. 2017;15(1):375–379. https://doi.org/10.3892/mmr.2016.6008
Wang H, Ye Y, Chu J-H, et al. Oridonin induces G2/M cell cycle arrest and apoptosis through MAPK and p53 signaling pathways in HepG2 cells. Oncol Rep. 2010;24(3):647–651. https://doi.org/10.3892/or_00000903
Gao S-Y, Li J, Qu X-Y, Zhu N, Ji Y-B. Downregulation of Cdk1 and cyclinB1 expression contributes to oridonin-induced cell cycle arrest at G 2/M phase and growth inhibition in SGC-7901 gastric cancer cells. Asian Pacific J Cancer Prevent. 2014;15(15):6437–6441.
Jin S, Shen J-n, Wang J, Huang G, Zhou J-G. Oridonin induced apoptosis through Akt and MAPKs signaling pathways in human osteosarcoma cells. Cancer Biol Ther. 2007;6(2):261–268. https://doi.org/10.4161/cbt.6.2.3621
Shen Q-K, Deng H, Wang S-B, Tian Y-S, Quan Z-S. Synthesis, and evaluation of in vitro and in vivo anticancer activity of 14-substituted oridonin analogs: a novel and potent cell cycle arrest and apoptosis inducer through the p53-MDM2 pathway. Eur J Med Chem. 2019;173:15–31. https://doi.org/10.1016/j.ejmech.2019.04.005
Jiang JH, Pi J, Jin H, Cai JY. Oridonin‐induced mitochondria‐dependent apoptosis in esophageal cancer cells by inhibiting PI3K/AKT/mTOR and Ras/Raf pathways. J Cell Biochem. 2019;120(3):3736–3746. https://doi.org/10.1002/jcb.27654
Cui Q, Yu J-h, Wu J-n, et al. P53-mediated cell cycle arrest and apoptosis through a caspase-3-independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells. Acta Pharm Sin. 2007;28(7):1057–1066. https://doi.org/10.1111/j.1745-7254.2007.00588.x
Yang IH, Shin JA, Lee KE, Kim J, Cho NP, Cho SD. Oridonin induces apoptosis in human oral cancer cells via phosphorylation of histone H2 AX. This study demonstrates that oridonin induces apoptosis in human oral cancer cells by promoting the phosphorylation of histone H2AX (γH2AX), a marker of DNA damage. Their work revealed that oridonin treatment led to reduced cell viability and significantly increased γH2AX expression in HSC-3 and HSC-4 oral cancer cell lines. Additionally, oridonin caused nuclear condensation and fragmentation, and induced cleavage of poly(ADP-ribose) polymerase (PARP). The accumulation of γH2AX was partially abrogated by Z-VAD, a pan-caspase inhibitor, indicating the involvement of caspase-dependent pathways in oridonin-induced apoptosis. These findings suggest that oridonin effectively induces apoptosis by enhancing γH2AX expression in response to DNA damage, positioning it as a promising candidate for oral cancer therapy. Eur J Oral Sci. 2017;125(6):438–443. https://doi.org/10.1111/eos.12387
Podhorecka M, Skladanowski A, Bozko P. H2AX phosphorylation: its role in DNA damage response and cancer therapy. J Nucleic Acids. 2010;2010. https://doi.org/10.4061/2010/920161
Mavragani IV, Nikitaki Z, Souli MP, et al. Complex DNA damage: a route to radiation-induced genomic instability and carcinogenesis. Cancers. 2017;9(7):e91. https://doi.org/10.3390/cancers9070091
Kwon H-J, Kim L-H, Ahn C-H, et al. A new insight into the apoptotic effect of nitidine chloride targeting checkpoint kinase 2 in human cervical cancer in vitro. J Clinic Biochem Nutrition. 2019:19–28. https://doi.org/10.3164/jcbn.19-28
Xu Z-Z, Fu W-B, Jin Z, Guo P, Wang W-F, Li J-M. Reactive oxygen species mediate oridonin-induced apoptosis through DNA damage response and activation of JNK pathway in diffuse large B cell lymphoma. Leuk Lymphoma. 2016;57(4):888–898. https://doi.org/10.3109/10428194.2015.1061127
Mah L, El-Osta A, Karagiannis T. γH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24(4):679–686. https://doi.org/10.1038/leu.2010.6
Rajendran P, Sekar R, Dhayasankar PS, et al. PI3K/AKT signaling pathway mediated autophagy in oral carcinoma-A comprehensive review. This comprehensive review focuses on the role of the PI3K/AKT signaling pathway in regulating autophagy in oral squamous cell carcinoma (OSCC). The authors critically analyze how dysregulation of this pathway contributes to tumor progression, resistance to apoptosis, and impaired autophagic flux. The review also explores therapeutic strategies targeting PI3K/AKT to restore autophagy and enhance cancer cell death. This updated synthesis highlights the central role of PI3K/AKT signaling in oral cancer biology and its potential as a therapeutic target. Int J Med Sci. 2024;21(6):1165–1175. https://doi.org/10.7150/ijms.94566
Xu J, Li Y, Kang M, et al. Multiple forms of cell death: a focus on the PI3K/AKT pathway. J Cell Physiol. 2023;238(9):2026–2038. https://doi.org/10.1002/jcp.31087
Song M, Liu X, Liu K, et al. Targeting AKT with oridonin inhibits growth of esophageal squamous cell carcinoma in vitro and patient-derived xenografts in vivo. This study investigates the anticancer efficacy of oridonin against esophageal squamous cell carcinoma (ESCC) and identifies AKT as a critical molecular target. The authors demonstrate that oridonin significantly inhibits ESCC cell proliferation, induces apoptosis, and impairs tumor growth in patient-derived xenograft models. Mechanistically, oridonin suppresses AKT phosphorylation and downstream signaling pathways involved in cell survival. The findings provide strong preclinical evidence supporting oridonin as a promising therapeutic agent for ESCC through the inhibition of AKT signaling. Mol Cancer Therapeut. 2018;17(7):1540–1553. https://doi.org/10.1158/1535-7163.MCT-17-0823
Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462.
Darling NJ, Cook SJ. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Bioch et Bioph Acta-Molecul Cell Res. 2014;1843(10):2150–2163. https://doi.org/10.1016/j.bbamcr.2014.01.009
Jalmi SK, Sinha AK. ROS mediated MAPK signaling in abiotic and biotic stress-striking similarities and differences. Front Plant Sci. 2015;6:e769. https://doi.org/10.3389/fpls.2015.00769
Allenspach EJ, Maillard I, Aster JC, Pear WS. Notch signaling in cancer. Cancer Biol Ther. 2002;1(5):466–476. https://doi.org/10.4161/cbt.1.5.159
Ali J, Sabiha B, Jan HU, Haider SA, Khan AA, Ali SS. Genetic etiology of oral cancer. Oral Oncol. 2017;70:23–28. https://doi.org/10.1016/j.oraloncology.2017.05.004
Osathanon T, Nowwarote N, Pavasant P. Expression and influence of Notch signaling in oral squamous cell carcinoma. J Oral Sci. 2016;58(2):283–294. https://doi.org/10.2334/josnusd.15-0535
Salameti V, Bhosale PG, Ames-Draycott A, Sipilä K, Watt FM. NOTCH1 signaling in oral squamous cell carcinoma via a TEL2/SERPINE1 axis. Oncotarget. 2019;10(63):6791–6804. https://doi.org/10.18632/oncotarget.27306
Dong Y, Zhang T, Li J, et al. Oridonin inhibits tumor growth and metastasis through anti-angiogenesis by blocking the Notch signaling. PLoS One. 2014;9(12):e113830. https://doi.org/10.1371/journal.pone.0113830
Yoshida R, Nagata M, Nakayama H, et al. The pathological significance of Notch1 in oral squamous cell carcinoma. Laborat Invest. 2013;93(10):1068–1081. https://doi.org/10.1038/labinvest.2013.95
Jahan S, Kumar D, Chaturvedi S, et al. Therapeutic targeting of NLRP3 inflammasomes by natural products and pharmaceuticals: a novel mechanistic approach for inflammatory diseases. Curr Med Chem. 2017;24(16):1645–1670. https://doi.org/10.2174/0929867324666170227121619
Ito M, Shichita T, Okada M, et al. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat Commun. 2015;6(1):e7360. https://doi.org/10.1038/ncomms8360
Inoue M, Shinohara ML. Nlrp3 inflammasome and MS/EAE. Auto Dis. 2013;2013. https://doi.org/10.1155/2013/859145
Halle A, Hornung V, Petzold GC, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol. 2008;9(8): 857–865. https://doi.org/10.1038/ni.1636
Schmid-Burgk JL, Chauhan D, Schmidt T, et al. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J Biol Chem. 2016;291(1):103–109. https://doi.org/10.1074/jbc.C115.700492
Feng X, Luo Q, Zhang H, et al. The role of NLRP3 inflammasome in 5-fluorouracil resistance of oral squamous cell carcinoma. This study explores the molecular mechanisms underlying chemoresistance in oral squamous cell carcinoma (OSCC), specifically focusing on the role of the NLRP3 inflammasome in modulating the response to 5-fluorouracil (5-FU). The authors demonstrate that the activation of the NLRP3 inflammasome contributes to 5-FU resistance by promoting an inflammatory tumor microenvironment and inhibiting apoptosis. Targeting the NLRP3 pathway may therefore serve as a promising therapeutic strategy to overcome drug resistance in OSCC. J Exp Clinic Cancer Res. 2017;36(1):e81. https://doi.org/10.1186/s13046-017-0553-x
Bae JY, Lee S-W, Shin Y-H, Lee J-H, Jahng JW, Park K. P2X7 receptor and NLRP3 inflammasome activation in head and neck cancer. Oncotarget. 2017;8(30):48972– 48982. https://doi.org/10.18632/oncotarget.16903
Qiu W, Chen R, Chen X, et al. Oridonin-loaded and GPC1-targeted gold nanoparticles for multimodal imaging and therapy in pancreatic cancer. Int J Nanomed. 2018;13:6809–6827.
Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflam Res. 2015;8:15–27.
Huang Y, Jiang H, Chen Y, et al. Tranilast directly targets NLRP 3 to treat inflammasome‐driven diseases. EMBO Mol Med. 2018;10(4):e8689.
Pan M-H, Chiou Y-S, Tsai M-L, Ho C-T. Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Complementary Med. 2011;1(1):8–24. https://doi.org/10.1016/S2225-4110(16)30052-9
Duncan JA, Bergstralh DT, Wang Y, et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Nat Acad Sci. 2007;104(19):8041–8046. https://doi.org/10.1016/S2225-4110(16)30052-9
He Y, Varadarajan S, Muñoz-Planillo R, Burberry A, Nakamura Y, Núñez G. 3, 4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem. 2014;289(2):1142–1150. https://doi.org/10.1074/jbc.M113.515080
Wu G, Guo Y, Liu Y, et al. Network‐Based method to investigate the promoted cell apoptosis mechanisms of oridonin in OSCC through the RNA‐Transcriptome. J Immunol Res. 2023;2023(1):e5293677. https://doi.org/10.1155/2023/5293677
Hu X, Wang Y, Gao X, et al. Recent progress of oridonin and its derivatives for the treatment of acute myelogenous leukemia. Mini Rev Med Chem. 2020;20(6):483–497. https://doi.org/10.2174/1389557519666191029121809
Zhao C-L, Zhang C-Y, Yang X-M, et al. Design and synthesis of oridonin derivatives as cytotoxic agents. Nat Prod Res. 2025;39(3):550–558. https://doi.org/10.1080/14786419.2023.2275287
Xu J, Wold EA, Ding Y, Shen Q, Zhou J. Therapeutic potential of oridonin and its analogs: from anticancer and antiinflammation to neuroprotection. Molecules. 2018;23(2):e474. https://doi.org/10.3390/molecules23020474
Pi J, Jiang J, Cai H, et al. GE11 peptide conjugated selenium nanoparticles for EGFR targeted oridonin delivery to achieve enhanced anticancer efficacy by inhibiting EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathways. Drug Deliver. 2017;24(1):1549–1564. https://doi.org/10.1080/10717544.2017.1386729
Copyright (c) 2025 Ome Kalsoom Afridi, Abdus Salam, Habib Ullah Jan, Abid Ali Khan, Johar Ali

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










