Exploring the Potential Anti-diabetic Properties of Rhapis excelsa through Computational Approaches
Abstract
 Abstract Views: 0
Abstract Views: 0
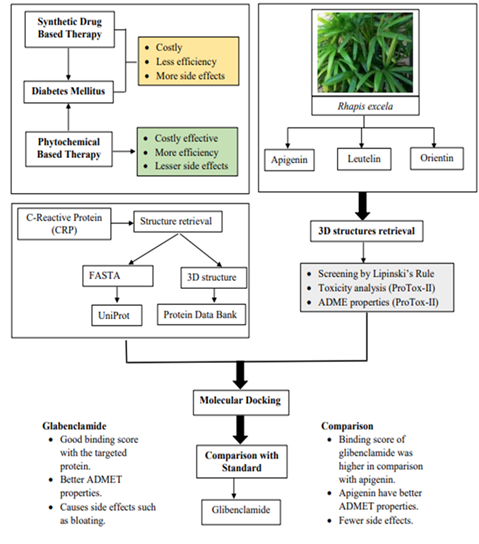
Background. Phytotherapy has been practiced against many acute and chronic diseases by different ethnic groups around the globe. Among these medicinal plants, the species of Arecaceae family stands out due to their vast economic importance. Most of the family members contain rich phytochemicals and secondary metabolites, being part of this family, Rhapis excelsa contains flavonoids, terpenoids, tannins, and many other bioactive metabolites which are believed to contribute to its medicinal properties, potentially offering therapeutic benefits against Diabetes mellitus which is a complex metabolic disorder that has become a major global health concern. The aim was to identify and select the potential bioactive compounds from Rhapis excelsa by using computational approaches for the treatment and management of DM.
Methods. C-reactive protein (CRP) was selected as the target protein. Three different ligands from Rhapis excelsa underwent both physicochemical and toxicity assessments using SwissADME and ProTox-II. Computational approaches, such as molecular docking and virtual screening were used to evaluate binding affinity.
Results. The leading compound identified was apigenin which was then compared to the standard anti-diabetic drug, Glibenclamide. The results indicated that apigenin and Glibenclamide have similar functions when selected as ligands against the target protein. Literature evidence also supports the anti-diabetic effects of apigenin.
Conclusion. The current study identified potential phytochemical compounds from Rhapis excelsa using computational approaches for their application in diabetes drug development. Computational analyses including molecular docking and virtual screening revealed some bioactive compounds within Rhapis excelsa.
Downloads
References
Jacob B, Narendhirakannan RT. Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech. 2018;9(1):e4. https://doi.org/10.1007/s13205-018-1528-0
This review article is written by Bindu Jacob and Narendhirakannan that is focused on the use of medicinal plants in the treatment and management of diabetes mellitus. Medicinal plants containing different phytochemicals are discussed in the article. Systematic search was performed by using different search engines such as Google Scholar, Science Direct, PubMed and different journals and books. These plants have anti-diabetic, anti-lipidemic and insulin mimetic properties that are required in diabetes management. To have a cost effective and improved therapeutic effects, medicinal plants are best choice.
Adhikari B. Roles of alkaloids from medicinal plants in the management of diabetes mellitus. J Chem. 2021;2021(1):e2691525. https://doi.org/10.1155/2021/2691525
This article is written by Bikash Adhikari that is mainly focused on the role of medicinal plants containing different phytochemicals specially alkaloids in diabetes mellitus. These are most sbundant secondary metabolized that are involve in inhibition of enzymes involve in post prandial hyperglycemia. Literature review was performed by using Google Scholar, Science Direct, PubMed, Springer and Scopus by using different key words such as hypoglycemia, anti-diabetic, alkaloids and medicinal plants. Alkaloids increase insulin excretion and sensitivity and increase glucose uptake in cells.
Han DG, Cho SS, Kwak JH, Yoon IS. Medicinal plants and phytochemicals for diabetes mellitus: pharmacokinetic characteristics and herb-drug interactions. J Pharm Invest. 2019;49(6):603–612. https://doi.org/10.1007/s40005-019-00440-4
This article is written by Dong-Gyun Han and others that is focused on the void present in treatment of diabetes mellitus. There are no ideal drug based therapy for diabetes mellitus. To fill this voids medicinal plants can be used that are cost effective and safe for use. Many bioactive compounds from various plants are considered effective and can modulate drug metabolizing enzymes most importantly cytochrome P450 and increases pharmacokinetic properties of the drug.
Singh P, Singh VK, Singh AK. Molecular docking analysis of candidate compounds derived from medicinal plants with type 2 diabetes mellitus targets. Bioinformation. 2019;15(3):179–188. https://doi.org/10.6026/97320630015179
Kottaisamy CPD, Raj DS, Kumar VP, Sankaran U. Experimental animal models for diabetes and its related complications—a review. Lab Anim Res. 2021;37:e23. https://doi.org/10.1186/s42826-021-00101-4
Suryasa IW, Rodríguez-Gámez M, Koldoris T. Health and treatment of diabetes mellitus. Int J Health Sci. 2021;5(1):e572192. https://doi.org/10.53730/ijhs.v5n1.2864
Meo SA, Zia I, Bukhari IA, Arain SA. Type 2 diabetes mellitus in Pakistan: current prevalence and future forecast. J Pak Med Assoc. 2016;66(12):1637–1642.
Aamir AH, Ul-Haq Z, Mahar SA, et al. Diabetes prevalence survey of Pakistan (DPS-PAK): prevalence of type 2 diabetes mellitus and prediabetes using HbA1c: a population-based survey from Pakistan. BMJ Open. 2019;9(2):e025300. https://doi.org/10.1136/bmjopen-2018-025300
Sangeetha R. Luteolin in the management of type 2 diabetes mellitus. Curr Res Nutr Food Sci J. 2019;7(2):393–398. http://dx.doi.org/10.12944/CRNFSJ.7.2.09
Kanmani S, Kwon M, Shin MK, Kim MK. Association of C-Reactive protein with risk of developing type 2 diabetes mellitus, and role of obesity and hypertension: a large population-based Korean cohort study. Sci Rep. 2019;9:e4573. https://doi.org/10.1038/s41598-019-40987-8
Miao L, Cheong MS, Zhou C, Farag M, Cheang WS, Xiao J. Apigenin alleviates diabetic endothelial dysfunction through activating AMPK/PI3K/Akt/eNOS and Nrf2/HO‐1 signaling pathways. Food Front. 2022;4(1):420–431. https://doi.org/10.1002/fft2.192
Vanaja D, Kavitha S. A study on phytochemicals, antioxidant activity and FT-IR analysis of Rhapis Excelsa (THUNB.) A. HENRY. Eur J Pharm Med Res. 2016:3(7):390–394.
Biya A. The role of high sensitivity C-reactive protein level in predicting stroke and other cardiovascular events: a meta-analysis review. Int J Health Pharm. 2024;9(4):104–128. https://doi.org/10.56201/ijhpr.v9.no4.2024.pg104.128
Stanimirovic J, Radovanovic J, Banjac K, et al. Role of C‐reactive protein in diabetic inflammation. Mediat Inflam. 2022;2022(1):e3706508. https://doi.org/10.1155/2022/3706508
Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucl Acids Res. 2018;46(W1):W257–W263. https://doi.org/10.1093/nar/gky318
Pollastri MP. Overview on the rule of five. Curr Protoc Pharmacol. 2010;49(1):9–12. https://doi.org/10.1002/0471141755.ph0912s49
Anup N, Gadeval A, Rajpoot K, Tekade RK. Software used in ADME computation. In: Tekade AK, ed. Biopharmaceutics and Pharmacokinetics Considerations. Elsevier; 2021:699–708. https://doi.org/10.1016/B978-0-12-814425-1.00006-1
Surhone LM, Timpledon MT, Marseken SF, eds. UCSF Chimera. Betascript Publishing; 2013
Copyright (c) 2025 Iqra Sagheer, Sania Riaz, Mahnoor Azhar, Mariam Tariq, Hamza Masood, Sharjeel Tariq

This work is licensed under a Creative Commons Attribution 4.0 International License.
BSR follows an open-access publishing policy and full text of all published articles is available free, immediately upon publication of an issue. The journal’s contents are published and distributed under the terms of the Creative Commons Attribution 4.0 International (CC-BY 4.0) license. Thus, the work submitted to the journal implies that it is original, unpublished work of the authors (neither published previously nor accepted/under consideration for publication elsewhere). On acceptance of a manuscript for publication, a corresponding author on the behalf of all co-authors of the manuscript will sign and submit a completed the Copyright and Author Consent Form.










