Unravelling the Impact of Hypoxia, Reactive Oxygen Species, and Necrosis in Skeletal Muscles
Abstract
 Abstract Views: 0
Abstract Views: 0
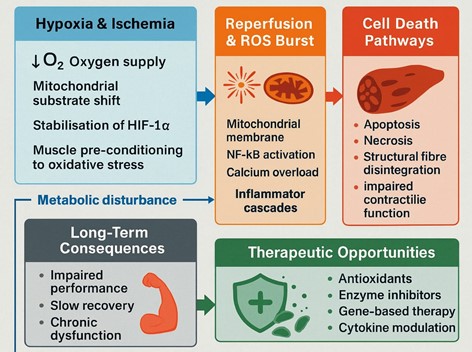
Skeletal muscle mass is strongly linked to stressors like ischemia and reactive oxygen species (ROS), both of which are regulated by oxygen availability and redox homeostasis. Intermittent ischemia and reperfusion cause a burst of reactive oxygen species, destruction of mitochondrial integrity, and inflammatory/necrotic pathways The objective of this review is to summarise existing data on the mechanistic interaction between hypoxia, ROS generation, and necrosis in skeletal muscle, as well as the role of these mechanisms in contributing to ischemia-reperfusion injury, metabolic disruption and dysfunction of skeletal muscle in the long term. The keywords examined in the literature search were skeletal muscle, hypoxia, reactive oxygen species, ischemia-reperfusion, mitochondria, and necrosis using PubMed, Scopus, and Web of Science. Articles published in English between 1990 and 2023 were peer reviewed and included, while conference abstracts, non-scientific reports, and duplicate records were excluded. The evidence suggests that hypoxia changes the use of substrates in the mitochondrion, stabilises the hypoxia-inducible factors and preconditions the muscle fibres to oxidative damage. The overproduction of ROS during reperfusion further increases the activity of inflammatory signalling, like NF-kB, calcium overload, and apoptotic and necrotic cell death. These teamed disruptions are what cause structural disintegration, dysfunctional contractional performance and retarded recuperation. An improved insight into these interrelated processes identifies prospects in therapeutic approaches such as antioxidants, enzymatic inhibition, gene-based treatment, cytokine therapy, and cell-derived exosomes- to alleviate ROS-related damage and promote muscle recovery.
Downloads
References
Powers SK, Morton AB, Ahn B, Smuder AJ. Redox control of skeletal muscle atrophy. Free Rad Biol Med. 2016;98:208-217. https://doi.org/10.1016/j.freeradbiomed.2016.02.021
Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524-551. https://doi.org/10.1016/j.redox.2015.08.020
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia–reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229-317. https://doi.org/10.1016/B978-0-12-394309-5.00006-7
Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10(6):620-630. https://doi.org/10.1016/S0967-2109(02)00070-4
Zuo L, Zhou T, Pannell BK, Ziegler AC, Best TM. Biological and physiological role of reactive oxygen species—the good, the bad, and the ugly. Acta Physiol. 2015;214(3):329-348. https://doi.org/10.1111/apha.12515
Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656-665. https://doi.org/10.1056/NEJMra0910283
Mason SD, Howlett RA, Kim MJ, et al. Loss of skeletal muscle HIF-1α results in altered exercise endurance. PLoS Biol. 2004;2(10):e288. https://doi.org/10.1371/journal.pbio.0020288
Jackson MJ. Reactive oxygen species and redox-regulation of skeletal muscle adaptations to exercise. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2285-2291. https://doi.org/10.1098/rstb 2005.1767
Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32(1):37-43. https://doi.org/10.1016/j.tibs.2006.11.001
Du J, Wang X, Miereles C, et al. Activation of caspase-3 is an initial step triggering muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113(1):115-123. https://doi.org/10.1172/JCI18330
Piccirillo R. Exercise-induced skeletal muscle adaptations: Redox signalling and the role of PGC-1α. Front Cell Dev Biol. 2020;8:e665. https://doi.org/10.3389/fcell.2020.00665
Hoek TLV, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia before reperfusion. J Mol Cell Cardiol. 1997;29(9):2571-2583. https://doi.org/10.1006/jmcc.1997.0464
Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol. 2017;17(3):165-178. https://doi.org/10.1038/nri.2016.150
Pisconti A, Dekempeneer L, Le Grand F. The emerging role of TNF-α in skeletal muscle development and regeneration. Skeletal Muscle. 2019;9(1):e1.
Chatzigeorgiou A, Halapas A, Kalafatakis K, Kamper EF. The role of cytokines in muscle damage and repair. Front Biosci. 2008;13:5144-5156. https://doi.org/10.2741/3083
Arif I, Ullah F. Impact of traffic stress, built environment, and socioecological factors on active transport among young adults. Sustainability. 2025;17(20):e9159. https://doi.org/10.3390/su17209159
Arif I, Jaweria A, Munir F. Health impacts of road traffic near school: narrative review. Pak Armed Forc Med J. 2025;75(1):209-216. https://doi.org/10.51253/pafmj.v75i
Song Y, Zhao X, Zhang J, et al. The role of VEGF in skeletal muscle regeneration. Front Physiol. 2021;12:e680220. https://doi.org/10.3389/fphys.2021.680220
Boppart MD, De Lisio M. Exercise and stem cells. Prog Mol Biol Transl Sci. 2015;135:423-456. https://doi.org/10.1016/bs.pmbts.2015.07.016
Arif I. Telehealth intervention of cardiovascular diseases patients: a quantitative study. Glob Drug Des Develop Rev. 2022;7(4):1-13. https://doi.org/10.31703/gdddr.2022(VII-IV).01
Bijlsma AY, Meskers CG, Westendorp RG, Maier AB. Chronology of muscle mass loss: Age-related muscle loss in humans. Age (Dordr). 2013;35(1):249-258. https://doi.org/10.1007/s11357-011-9342-9
Khan Y, Yablon LM, Rios CN, et al. Low-level laser therapy improves muscle regeneration in an ischemia-reperfusion model. Lasers Med Sci. 2020;35(3):727-736. https://doi.org/10.1007/s10103-019-02863-2
Llewellyn ME, Thompson KR, Deisseroth K, Delp SL. Orderly recruitment of motor units under optical control in vivo. Nat Med. 2010;16(10):1161-1165. https://doi.org/10.1038/nm.2228
Hurley BF, Hanson ED, Sheaff AK. Strength training as a countermeasure to ageing muscle: efects on muscle mass, strength, and function. Sports Med. 2011;41(4):289-306. https://doi.org/10.2165/11585920-000000000-00000
Yu T, Robergs RA, Yang Y. Exercise-induced mitochondrial stress: a brief review. Sports Med Health Sci. 2022;4(2):68-74. https://doi.org/10.1016/j.smhs.2022.03.001
O'Leary MF, Hood DA. Denervation-induced oxidative stress and autophagy in skeletal muscle. Autophagy. 2009;5(2):230-231. https://doi.org/10.4161/auto.5.2.7424
Liu L, Cheung WF, Li B, Chen S. Stem cell-based therapies for muscle regeneration: Current challenges and future directions. Stem Cells Int. 2022;2022:1-18. https://doi.org/10.1155/2022/7440039
Liu X, Wang D, Zhang X, Li Y, Li L. Role of microRNAs in skeletal muscle atrophy. Front Physiol. 2021;12:e685087. https://doi.org/10.3389/fphys.2021.685087
Arif I. Determinants of happiness among university students: a cross-sectional study in Pakistan. Glob Soc Sci Rev. 2024 ;9(1):248-256. http://dx.doi.org/10.31703/gssr.2024(IX-I).22
Arif I. Fundamental stratification of communication to overcome health inequities. Glob Immunol Infect Dis Rev. 2024;9(2):1-7. http://dx.doi.org/10.31703/giidr.2024(IX-II).01
Copyright (c) 2025 Farwa Munir, Farooq Manzoor, Shahzaib Naeem, Emad Abdulrahman H. Alsaedi, Maryum Hamayoun, Sher Wali Khan, Atif Amin Baig

This work is licensed under a Creative Commons Attribution 4.0 International License.





