A Structure-Based Computational Vaccine Strategy for the Emerging Isfahan Virus: In-Silico Vaccine Designing
Abstract
 Abstract Views: 0
Abstract Views: 0
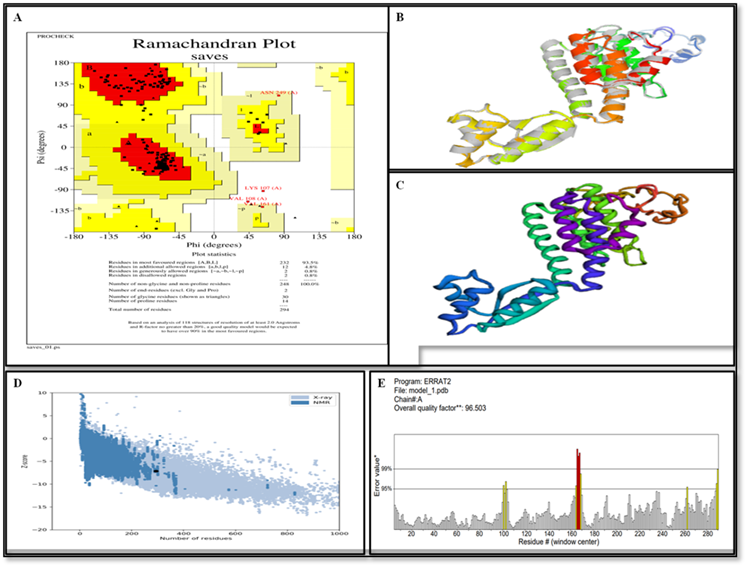
Isfahan virus (ISFV) is a recently discovered zoonotic disorder that causes painful and severe neurological effects in humans. The virus was initially identified after an epidemic in horses and cattle in 1916, and was later isolated from cattle in Richmond, Indiana in 1925. Currently, no effective vaccine is available for ISFV virus. Therefore, the current work aimed to generate a multi-epitope-based vaccine (MEV) candidate targeting Isfahan virus glycoprotein and large polymerase protein. From these proteins, nine epitopes (three T-cell and B-cell epitopes) were finally designated based on their non-allergenic, non-toxic, and antigenic properties. The selected epitopes, together by suitable adjuvant, enabled the development of an antigenic ISFV-MEV candidate free from allergic reactions. Computational modeling showed that the designed MEV binding strongly interacted with human TLR-3 receptors. Furthermore, immune simulations research established that the ISFV-MEV candidate has the potential to elicit robust immune system reactions in humans. Overall, the outcomes of these in silico studies are promising; however, a subsequent in vivo validation is recommended to confirm its potential as a future vaccine candidate.
Downloads
References
Knipe DM, Howley PM, eds. Fields Virology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
Tesh RB, Travassos da Rosa APA, Travassos da Rosa JFS. Antigenic relationship among rhabdoviruses infecting terrestrial vertebrates. J Gen Virol. 1983;64(1):169-176. https://doi.org/10.1099/0022-1317-64-1-169
Sia G, Tesh RB, Travassos da Rosa APA, et al. Isolation of the Isfahan virus in Turkmenia. Acta Virol. 1980;24(5):618-620.
Tesh RB, Travassos da Rosa APA, Travassos da Rosa JFS. Isfahan virus, a new vesiculovirus infecting humans, gerbils, and sandflies in Iran. Am J Trop Med Hyg. 1977;26(2):299-306. https://doi.org/10.4269/ajtmh.1977.26.299
Wilks CR, House JA. Susceptibility of various animals to the vesiculoviruses Isfahan and Chandipura. Epidemiol Infect. 1986;97(2):359-368. https://doi.org/10.1017/S002217240006544X
Travassos da Rosa JFS, Tesh RB. Isfahan virus, a new vesiculovirus infecting, gerbils, and sandflies. J Trop Med. 1982;29(1):291-302.
Nasar F, Palacios G, Gorchakov RV, et al. Recombinant Isfahan virus and vesicular stomatitis virus vaccine vectors provide durable multivalent single-dose protection against lethal alphavirus challenge. J Virol. 2017;91(8):e01729-16. https://doi.org/10.1128/JVI.01729-16
Cotton WE. The causal agent of vesicular stomatitis proved to be a filter-passing virus. J Am Vet Med Assoc. 1926;69:168-179.
Letchworth GJ, Rodriguez LL, Del Cbarrera J. Vesicular stomatitis. Vet J. 1999;157(3):239-260. https://doi.org/10.1053/tvjl.1998.0303
Johnson KM, Vogel JE, Peralta PH. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus. Am J Trop Med Hyg. 1966;15(2):244-246. https://doi.org/10.4269/ajtmh.1966.15.244
Fields BN, Hawkins K. Human infection with the virus of vesicular stomatitis during an epizootic. N Engl J Med. 1967;277(19):989-994. https://doi.org/10.1056/NEJM196711092771901
Howley PM, Knipe DM, eds. Fields Virology: Emerging Viruses. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2020.
Ball LA, White CN. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci USA. 1976;73(2):442-446. https://doi.org/10.1073/pnas.73.2.442
Ge P, Tsao J, Schein S, et al. Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science. 2010;327(5966):689-693. https://doi.org/10.1126/science.1181766
Whelan SPJ, Barr JN, Wertz GW. Transcription and replication of nonsegmented negative-strand RNA viruses. Curr Top Microbiol Immunol. 2004;283:61-119.
Ferris NP, Nordengrahn A, Hutchings GH, et al. Development and laboratory evaluation of two lateral flow devices for detection of vesicular stomatitis virus in clinical samples. J Virol Methods. 2012;180(1-2):96-100. https://doi.org/10.1016/j.jviromet.2011.12.010
McCluskey BJ, Peters D, Hannemann R, et al. Vesicular stomatitis outbreak in the southwestern United States, 2012. J Vet Diagn Invest. 2013;25(5):608-613. https://doi.org/10.1177/1040638713497945
Perez AM, Pauszek SJ, Jimenez D, et al. Spatial and phylogenetic analysis of vesicular stomatitis virus overwintering in the United States. Prev Vet Med. 2010;93(4):258-264. https://doi.org/10.1016/j.prevetmed.2009.11.003
Tolardo AL, Gómez MM, Fernandes M, et al. Real-time reverse transcriptase polymerase chain reaction for detection and quantification of vesiculovirus. Mem Inst Oswaldo Cruz. 2016;111:385-390. https://doi.org/10.1590/0074-02760150456
Maia-Farias A, Carvalho JG, Barros-Aragão FGQ, et al. Early and late neuropathological features of meningoencephalitis associated with Maraba virus infection. Braz J Med Biol Res. 2020;53:e9316. https://doi.org/10.1590/1414-431X20208604
Tosh RB, Chaniotis BN, Johnson KM. Vesicular stomatitis virus Indiana serotype: multiplication in and transmission by experimentally infected phlebotomine sandflies (Lutzomyia trapidoi). Am J Epidemiol. 1971;93(6):491-495. https://doi.org/10.1093/oxfordjournals.aje.a121284
Ajamma YU, Onchuru TO, Ouso DO, et al. Vertical transmission of naturally occurring bunyamwera and insect-specific flavivirus infections in mosquitoes from islands and mainland shores of Lakes Victoria and Baringo, Kenya. PLoS Negl Trop Dis. 2018;12(11):e0006949. https://doi.org/10.1371/journal.pntd.0006949
Calisher CH, Gutierrez E, Manzione N, et al. A newly recognized vesiculovirus, Calchaqui virus, and subtypes of Melao and Maguari viruses from Argentina, with serologic evidence for human and equine infection. Am J Trop Med Hyg. 1987;36(1):114-119. https://doi.org/10.4269/ajtmh.1987.36.114
Jonkers AH. The epizootiology of the vesicular stomatitis viruses: a reappraisal. Am J Epidemiol. 1976;104(3):286-291.
Shabbir MA, Ahmad I, Haq IU, et al. Immunoinformatics-driven design of a multi-epitope vaccine against Nipah virus. J Genet Eng Biotechnol. 2025;23(2):e100482. https://doi.org/10.1016/j.jgeb.2025.100482
Hakami MA. Immunoinformatics and structural vaccinology approach to design a multi-epitope vaccine targeting Zika virus. BMC Chem. 2024;18(1):e31.
Deb D, Srivastava R, Kumar S, et al. Immunoinformatics-based design of a multi-epitope vaccine against Chandipura vesiculovirus. J Cell Biochem. 2022;123(2):322-346.
Obaidullah AJ, Ahmed I, Khan T, et al. Immunoinformatics-guided design of a multi-epitope vaccine against SARS-CoV-2. RSC Adv. 2021;11(29):18103-18121.
Vita R, Overton JA, Greenbaum JA, et al. The immune epitope database (IEDB) 3.0. Nucleic Acids Res. 2015;43(Database issue):D405-D412. https://doi.org/10.1093/nar/gku938
Haq IU, Ahmad I, Shabbir MA, et al. Computational immunoinformatics approach to design a multi-epitope vaccine against Guanarito virus. World J Biol Biotechnol. 2025;10(1):25-35. https://doi.org/10.33865/wjb.10.1.1473
Doytchinova IA, Flower DR. Identifying candidate subunit vaccines using an alignment-independent method based on principal amino acid properties. Vaccine. 2007;25(5):856-866. https://doi.org/10.1016/j.vaccine.2006.09.032
Larsen MV, Lundegaard C, Lamberth K, et al. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinformatics. 2007;8:e424. https://doi.org/10.1186/1471-2105-8-424
Bibi B, Ahmad I, Haq IU, et al. Designing a multi-epitope-based vaccine against rubella virus using immunoinformatics approaches. Microbe. 2025;7:e100323. https://doi.org/10.1016/j.microb.2025.100323
Ahmad I, Haq IU, Shabbir MA, et al. Development of a multi-epitope subunit vaccine for protection against norovirus infections using computational vaccinology. J Biomol Struct Dyn. 2022;40(7):3098-3109.
Haq IU, Ahmad I, Shabbir MA, et al. Rational in silico design of a multi-epitope vaccine against human rhinovirus using immune simulation and molecular dynamics approaches. Vacunas. 2025;26:e500427.
Saha S, Raghava GPS. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins. 2006;65(1):40-48. https://doi.org/10.1002/prot.21078
Goel D, Bhatnagar R. Intradermal immunization with outer membrane protein 25 protects BALB/c mice from virulent Brucella abortus 544. Mol Immunol. 2012;51(2):159-168. https://doi.org/10.1016/j.molimm.2012.02.126
Goel D, Rajendran V, Bhatnagar R. Cell-mediated immune response after challenge in Omp25 liposome-immunized mice contributes to protection against virulent Brucella abortus 544. Vaccine. 2013;31(8):1231-1237. https://doi.org/10.1016/j.vaccine.2012.12.043
Rahiyab M, Ahmad I, Shabbir MA, et al. Design of a new multi-epitope subunit vaccine to combat the EIA virus, targeting Pol, Gag, and Env proteins: in silico technique. Vacunas. 2025;26:e500463. https://doi.org/10.1016/j.vacun.2025.500463
Khan A, Ahmad I, Haq IU, et al. Designing a highly antigenic multi-epitope subunit vaccine against bovine alphaherpesvirus 2 targeting glycoproteins B and H. Med Omics. 2025;8:e100047.
Hon J, Marusiak M, Martinek T, et al. SoluProt: prediction of protein solubility. Bioinformatics. 2019;35(9):1462-1464. https://doi.org/10.1093/bioinformatics/bty945
Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. 1999;292(2):195-202. https://doi.org/10.1006/jmbi.1999.3091
Deléage G. ALIGNSEC: viewing protein secondary structure predictions within large multiple sequence alignments. Bioinformatics. 2017;33(24):3991-3992. https://doi.org/10.1093/bioinformatics/btx577
Hussain I, Ahmad I, Haq IU, et al. Structure-based in silico discovery of thymidine kinase inhibitors targeting goatpox virus. ChemistrySelect. 2025;10(37):e03462. https://doi.org/10.1002/slct.202503462
Haq IU, Ahmad I, Shabbir MA, et al. Using immunoinformatics to design a rational in silico vaccine against human astrovirus targeting capsid polyprotein VP90. In Silico Pharmacol. 2025;13(3):e21.
Khan Z, Ahmad I, Shabbir MA, et al. In silico multi-epitope vaccine candidate against type 1 parainfluenza virus. Comput Biol Med. 2023;154:e106601. https://doi.org/10.21203/rs.3.rs-2455059/v1
Wiederstein M, Sippl MJ. ProSA-web: interactive web service for recognition of errors in three-dimensional protein structures. Nucleic Acids Res. 2007;35:W407-W410. https://doi.org/10.1093/nar/gkm290
Sippl MJ, Lackner P, Domingues FS, et al. Assessment of the CASP4 fold recognition category. Proteins. 2001;45(S5):55-67. https://doi.org/10.1002/prot.10006
Khan B, Ahmad I, Haq IU, et al. Systematic identification of molecular biomarkers and drug candidates targeting MAPK3 in multiple sclerosis. Hum Gene. 2025;12:e201436. https://doi.org/10.1016/j.humgen.2025.201436
Rahiyab M, Ahmad I, Shabbir MA, et al. Computational profiling of molecular biomarkers in congenital disorders of glycosylation type I and binding analysis of ginkgolide A with P4HB. Comput Biol Med. 2025;190:e110042. https://doi.org/10.1016/j.compbiomed.2025.110042
Shey RA, Ghogomu SM, Esoh KK, et al. In silico design of a multi-epitope vaccine candidate against onchocerciasis. Sci Rep. 2019;9(1):e4409. https://doi.org/10.1038/s41598-019-40833-x
Alizadeh M, Amini-Khoei H, Ahmad I, et al. Designing a novel multi-epitope vaccine against Ebola virus using reverse vaccinology. Sci Rep. 2022;12(1):e7757. https://doi.org/10.1038/s41598-022-11851-z
Ponomarenko J, Bui HH, Li W, et al. ElliPro: a new structure-based tool for prediction of antibody epitopes. BMC Bioinformatics. 2008;9:e514. https://doi.org/10.1186/1471-2105-9-514
Xagorari A, Chlichlia K. Toll-like receptors and viruses: induction of innate antiviral immune responses. Open Microbiol J. 2008;2:49-59. https://doi.org/10.2174/1874285800802010049
Yan Y, Tao H, He J, Huang SY. The HDOCK server for integrated protein–protein docking. Nat Protoc. 2020;15(5):1829-1852. https://doi.org/10.1038/s41596-020-0312-x
Ahmad S, Ahmad I, Haq IU, et al. Exploring the potential mechanism of Tinospora cordifolia in cancer treatment using network pharmacology and molecular docking. In Silico Res Biomed. 2025;4:e100124.
López-Blanco JR, Aliaga JI, Quintana-Ortí ES, Chacón P. iMODS: internal coordinates normal mode analysis server. Nucleic Acids Res. 2014;42(Web Server issue):W271-W276. https://doi.org/10.1093/nar/gku339
Rice P, Longden I, Bleasby A. EMBOSS: the European molecular biology open software suite. Trends Genet. 2000;16(6):276-277. https://doi.org/10.1016/S0168-9525(00)02024-2
Ahmad S, Haq IU, Shabbir MA, et al. Design of a novel multi-epitope vaccine against human torovirus disease using immunoinformatics. Comput Biol Chem. 2024;113:e108213. https://doi.org/10.1016/j.compbiolchem.2024.108213
Grote A, Hiller K, Scheer M, et al. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005;33:W526-W531. https://doi.org/10.1093/nar/gki376
Samad A, Ahmad I, Haq IU, et al. Designing a multi-epitope vaccine against SARS-CoV-2 using an immunoinformatics approach. J Biomol Struct Dyn. 2022;40(1):14-30.
Khan S, Ahmad I, Haq IU, et al. Exploring stevioside binding affinity with proteins involved in diabetic signaling pathways: an in silico approach. Front Pharmacol. 2024;15:e1377916. https://doi.org/10.3389/fphar.2024.1377916
Abdi SAH, Ahmad I, Haq IU, et al. Multi-epitope-based vaccine candidate for monkeypox virus: an in silico approach. Vaccines. 2022;10(9):1564. https://doi.org/10.3390/vaccines10091564
Souod N, Ahmad I, Haq IU, et al. In silico design and evaluation of a multiepitope vaccine against Bordetella pertussis. Genomics Inform. 2025;23:e16.
Sethi G, Ahmad I, Haq IU, et al. Designing a broad-spectrum multi-epitope subunit vaccine against leptospirosis using immunoinformatics. Front Immunol. 2025;15:e1503853. https://doi.org/10.3389/fimmu.2024.1503853
Yang Z, Bogdan P, Nazarian S. An in silico deep learning approach to multi-epitope vaccine design: a SARS-CoV-2 case study. Sci Rep. 2021;11(1):e3238. https://doi.org/10.1038/s41598-021-81749-9
Khan S, Ahmad I, Haq IU, et al. Developing a novel computational strategy for a multi-epitope vaccine against Guanarito virus. J Emerg Trends Novel Res. 2024;11(1):1-12.
Foroogh N, Ahmad I, Haq IU, et al. Structural and functional characterization of the FimH adhesin of uropathogenic Escherichia coli. J Struct Biol. 2021;161:e105288. https://doi.org/10.1016/j.micpath.2021.105288
Narula A, Pandey RK, Khatoon N, et al. Excavating chikungunya genome to design B- and T-cell multi-epitope subunit vaccine using immunoinformatics. Infect Genet Evol. 2018;61:4-15. https://doi.org/10.1016/j.meegid.2018.02.001
Alizadeh M, Ahmad I, Haq IU, et al. Designing a novel multi-epitope vaccine against Ebola virus using reverse vaccinology. Sci Rep. 2022;12(1):e7757.
Roundy CM, Azar SR, Rossi SL, Weaver SC. Insect-specific viruses: a historical overview and recent developments. Adv Virus Res. 2017;98:119-146. https://doi.org/10.1016/bs.aivir.2016.10.001
Bettis AA, Jackson ML, Yoon IK, et al. The global epidemiology of chikungunya from 1999 to 2020. PLoS Negl Trop Dis. 2022;16(1):e0010069. https://doi.org/10.1371/journal.pntd.0010069
Hierlihy C, Zambrano H, Alarcon-Elbal PM, et al. Individual and community mitigation measures for chikungunya virus prevention: a systematic review. PLoS One. 2019;14(2):e0212054. https://doi.org/10.1371/journal.pone.0212054
Romano P, Giugno R, Pulvirenti A. Tools and collaborative environments for bioinformatics research. Brief Bioinform. 2011;12(6):549-561. https://doi.org/10.1093/bib/bbr055
Robson B, Boray S. Web-based universal exchange and inference language for medicine. Comput Biol Med. 2015;66:82-102. https://doi.org/10.1016/j.compbiomed.2015.07.015
An W, Liu Y, Wang X, et al. Recent progress on chikungunya virus research. Virol Sin. 2017;32:441-453. https://doi.org/10.1007/s12250-017-4072-x
Fros JJ, van der Maten E, Vlak JM, Pijlman GP. Chikungunya virus nsP2 inhibits type I/II interferon-stimulated JAK–STAT signaling. J Virol. 2010;84(20):10877-10887. https://doi.org/10.1128/JVI.00949-10
Bae S, Lee JY, Myoung J. Chikungunya virus proteins nsP2, E2, and E1 antagonize interferon-β signaling. Viruses. 2019;11(7):e584. https://doi.org/10.3390/v11070584
Göertz GP, McNally KL, Robertson SJ, et al. The methyltransferase-like domain of chikungunya virus nsP2 inhibits interferon response. J Virol. 2018;92(17):e01008-18. https://doi.org/10.1128/JVI.01008-18
Schrauf S, Tschismarov R, Tauber E, et al. Current efforts in vaccine development for Zika and chikungunya virus infections. Front Immunol. 2020;11:e592. https://doi.org/10.3389/fimmu.2020.00592
Khan A, Ahmad I, Haq IU, et al. Allosteric ligands targeting flavivirus NS5 identified from ZINC database. J Mol Graph Model. 2018;82:37-47.
Pandey RK, Sundar S, Prajapati VK. Differential expression of miRNAs regulates T-cell differentiation during visceral leishmaniasis. Front Microbiol. 2016;7:206. https://doi.org/10.3389/fmicb.2016.00206
Aslam S, Ahmad I, Haq IU, et al. Designing a multi-epitope vaccine against Chlamydia trachomatis. Biology (Basel). 2021;10(10):e997. https://doi.org/10.3390/biology10100997
Mahmud S, Ahmad I, Haq IU, et al. Designing a multi-epitope vaccine candidate against MERS-CoV. Sci Rep. 2021;11(1):e15431.
Kadam A, Sasidharan S, Saudagar P. Computational design of a multi-epitope vaccine against Ebola virus. Infect Genet Evol. 2020;85:e104464. https://doi.org/10.1016/j.meegid.2020.104464
Shankar U, Gupta A, Kumar R, et al. Mining Ebola virus genome for multi-epitope vaccine construction. J Biomol Struct Dyn. 2022;40(11):4815-4831.
Qamar MT, Ahmad S, Fatima I, et al. Designing a multi-epitope vaccine against Staphylococcus aureus. Comput Biol Med. 2021;132:e104389. https://doi.org/10.1016/j.compbiomed.2021.104389
Solanki V, Tiwari V. Subtractive proteomics and reverse vaccinology for chimeric vaccine design against Acinetobacter baumannii. Sci Rep. 2018;8(1):e9044.
Gasteiger E, Hoogland C, Gattiker A, et al. Protein identification and analysis tools on the ExPASy server. In: Walker JM, ed. The Proteomics Protocols Handbook. Totowa, NJ: Humana Press; 2005:571-607.
Hussain I, Ahmad I, Haq IU, et al. Structure-guided drug repurposing reveals antiviral candidates for Bourbon virus. In Silico Pharmacol. 2025;13(3):155. https://doi.org/10.1007/s40203-025-00443-0
Khan S, Ahmad I, Haq IU, et al. Design of a novel multi-epitope vaccine against Bundibugyo ebolavirus. Med Omics. 2025;8:e100050. https://doi.org/10.1016/j.medomic.2025.100050
Morla S, Makhija A, Kumar S. Synonymous codon usage patterns in rabies virus glycoprotein gene. Gene. 2016;584(1):1-6. https://doi.org/10.1016/j.gene.2016.02.047
Brinton MA. The molecular biology of West Nile virus. Annu Rev Microbiol. 2002;56:371-402.
Kar T, Narsaria U, Basak S, et al. A candidate multi-epitope vaccine against SARS-CoV-2. Sci Rep. 2020;10(1):e10895. https://doi.org/10.1038/s41598-020-67749-1
Safavi A, Kefayat A, Mahdevar E, et al. Exploring hidden antigens of SARS-CoV-2 to design a multi-epitope vaccine. Vaccine. 2020;38(48):7612-7628. https://doi.org/10.1016/j.vaccine.2020.10.016
Copyright (c) 2025 Sajjad Ahmad, Sidra Amin, Muhammad Rahiyab, Rooh-Ullah, Arshad Iqbal, Syed Shujait Ali, Liaqat Ali, Salman Khan, Hyat Khan, Zahid Hussain

This work is licensed under a Creative Commons Attribution 4.0 International License.





