Comparative Assessment of Adams-Bashforth-Moulton, 4th order Runge-Kutta, and Euler Methods for the Synthesis of Zinc Oxide Nanostructures via the Lengyel Epstein Reaction Model
Abstract
 Abstract Views: 0
Abstract Views: 0
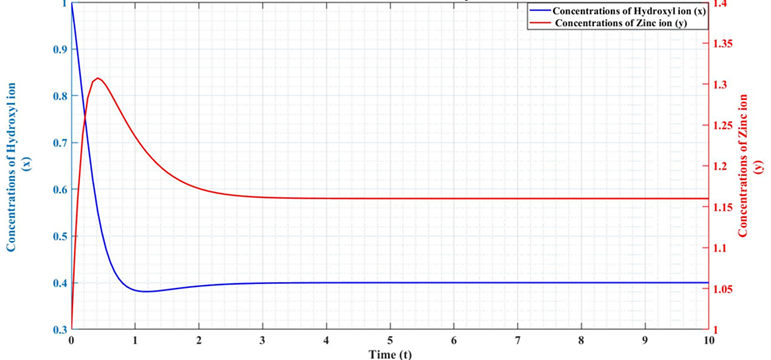
The current study aimed to determine which approximation technique is the most successful in studying the rise in ion concentrations in forms of Zinc Oxide (ZnO) nanostructures using the Lengyel Epstein Reaction Model. To achieve this objective, Ordinary Differential Equations (ODEs) were formulated utilizing three separate numerical methods. These included Euler, Adams-Bashforth-Moulton (ABM), and 4th Order Runge-Kutta (RK) methods. The current study aimed to identify the optimal approximation approach for computing concentrations of zinc ions Zn+2 and hydroxyl ions OH- while examining the reaction kinetics of ZnO nanostructures. The research findings indicated that the ABM approach surpasses the Euler and RK methods, convergence speed, and reduced error relative to the Euler and RK methods. The ABM approach further verifies experimental findings about ZnO nanostructure synthesis by the aqueous chemical growth (ACG) process, that affirms its efficacy practically
Downloads
References
Ali B, Khan AA. Real-time distribution system analysis and load management algorithm for minimizing harmonics. Roman J Tech Sci Electrotech Ene Ser. 2021;66(4):237-242.
Siddiqi KS, Rahman AU, Tajuddin N, Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett. 2018;13:1-13.
Tang E, Cheng G, Ma X, Pang X, Zhao Q. Surface modification of zinc oxide nanoparticle by PMAA and its dispersion in aqueous system. Appl Surf Sci. 2006;252(14):5227-5232. https://doi.org/10.1016/j.apsusc.2005.08.004
Jiang J, Pi J, Cai J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl. 2018;2018(1):e1062562. https://doi.org/10.1155/2018/1062562
Fatima K, Khan A, Hussain M. Mathematical modelling of reaction kinematics of one dimensional zinc oxide nanostructures. NED Univ J Res. 2018;15(4):117-122.
Mishra PK, Mishra H, Ekielski A, Talegaonkar S, Vaidya B. Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today. 2017;22(12):1825-1834. https://doi.org/10.1016/j.drudis.2017.08.006
Sabir S, Arshad M, Chaudhari SK. Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. Sci World J. 2014;2014(1):e925494. https://doi.org/10.1155/2014/925494
Osman DAM, Mustafa MA. Synthesis and characterization of zinc oxide nanoparticles using zinc acetate dihydrate and sodium hydroxide. J Nanosci Nanoeng. 2015;1(4):248-251.
Chandiran AK, Abdi-Jalebi M, Nazeeruddin MK, Grätzel M. Analysis of electron transfer properties of ZnO and TiO2 photoanodes for dye-sensitized solar cells. ACS Nano. 2014;8(3):2261-2268. https://doi.org/10.1021/nn405535j
Bai X, Wang L, Zong R, Lv Y, Sun Y, Zhu Y. Performance enhancement of ZnO photocatalyst via synergic effect of surface oxygen defect and graphene hybridization. Langmuir. 2013;29(9):3097-3105. https://doi.org/10.1021/la4001768
Hatamie A, Khan A, Golabi M, et al. Zinc oxide nanostructure-modified textile and its application to biosensing, photocatalysis, and as antibacterial material. Langmuir. 2015;31(39):10913-10921. https://doi.org/10.1021/acs.langmuir.5b02341
Fatima K, Ali B, Mahnoor. Implementation of Lengyel-Epstein reaction model for Zinc Oxide (ZnO) nanostructures by comparing euler and fourth-order Runge–Kutta (RK) Methods. Sci Inq Rev. 2022;6(1):23-33. https://doi.org/10.32350/sir.61.02
Ruby T, Azis D, Sutrisno A, Falen, Andrade PMF. Application of Adams-Bashforth-Moulton method for predict population growth in Banten province with logistic equation. Int J Adv Soc Sci Educ. 2025;3(1):73-86.
Cardoso D, Narcy A, Durosoy S, Bordes C, Chevalier Y. Dissolution kinetics of zinc oxide and its relationship with physicochemical characteristics. Powder Technol. 2021;378:746-759. https://doi.org/10.1016/j.powtec.2020.10.049
Kaur Y, Jasrotia T, Kumar R, Chaudhary GR, Chaudhary S. Adsorptive removal of eriochrome black T (EBT) dye by using surface active low cost zinc oxide nanoparticles: a comparative overview. Chemosphere. 2021;278:e130366. https://doi.org/10.1016/j.chemosphere.2021.130366
Khan A, Hussain M, Abbasi MA, Ibupoto ZH, Nur O, Willander M. Analysis of junction properties of gold–zinc oxide nanorods-based Schottky diode by means of frequency dependent electrical characterization on textile. J Mater Sci. 2014;49(9):3434-3441. https://doi.org/10.1007/s10853-014-8053-2
Qiu Y, Zhang H, Hu L, et al. Flexible piezoelectric nanogenerators based on ZnO nanorods grown on common paper substrates. Nanoscale. 2012;4(20):6568-6573. https://doi.org/10.1039/C2NR31031G
Lee CY, Wang J, Chou Y, et al. White-light electroluminescence from ZnO nanorods/polyfluorene by solution-based growth. Nanotechnology. 2009;20(42):e425202. https://doi.org/10.1088/0957-4484/20/42/425202
Greene LE, Yuhas BD, Law M, Zitoun D, Yang P. Solution-grown zinc oxide nanowires. Inorg Chem. 2006;45(19):7535-7543. https://doi.org/10.1021/ic0601900
Chicone C. Mathematical Modeling and Chemical Kinetics. University of Missouri: 2010.
Caetano BL, Santilli CV, Meneau F, Briois V, Pulcinelli SH. In situ and simultaneous UV- vis/SAXS and UV- vis/XAFS time-resolved monitoring of ZnO quantum dots formation and growth. J Phys Chem C. 2011;115(11):4404-4412. https://doi.org/10.1021/jp109585t
Mishra YK, Modi G, Cretu V, et al. Direct growth of freestanding ZnO tetrapod networks for multifunctional applications in photocatalysis, UV photodetection, and gas sensing. ACS Appl Mater Interfaces. 2015;7(26):14303-14316. https://doi.org/10.1021/acsami.5b02816
Copyright (c) 2025 Kaniz Fatima, Sarwat Ishaque, Basit Ali, Asif Sumeer, Qaiser Hafeez

This work is licensed under a Creative Commons Attribution 4.0 International License.






